Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(5); 2023 > Article
-
Brief Report
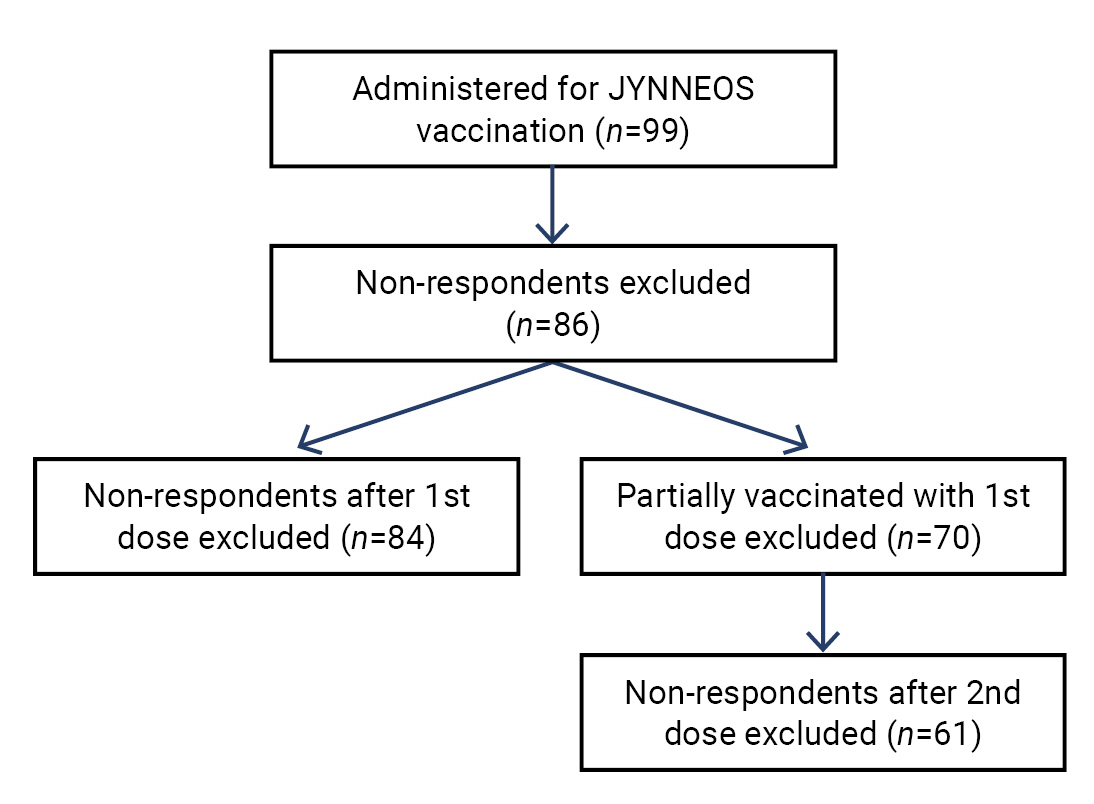
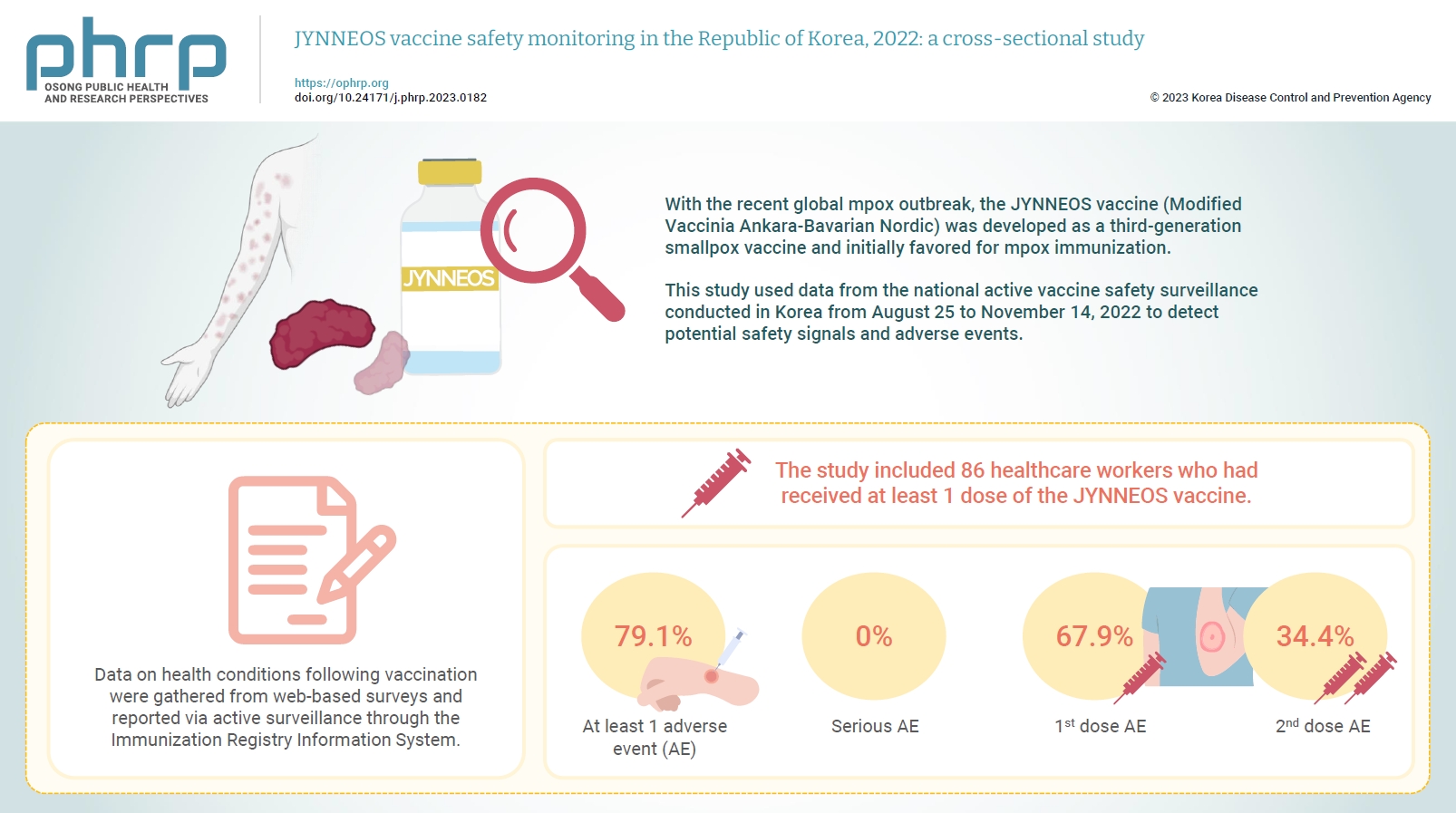
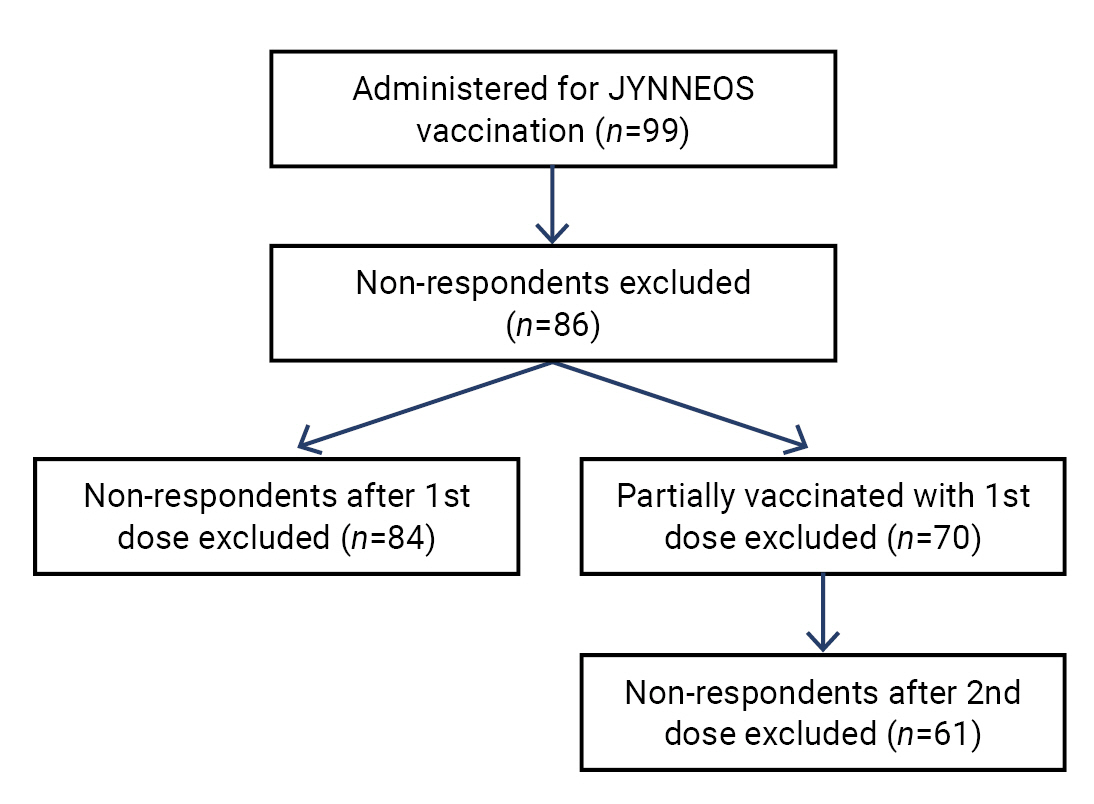
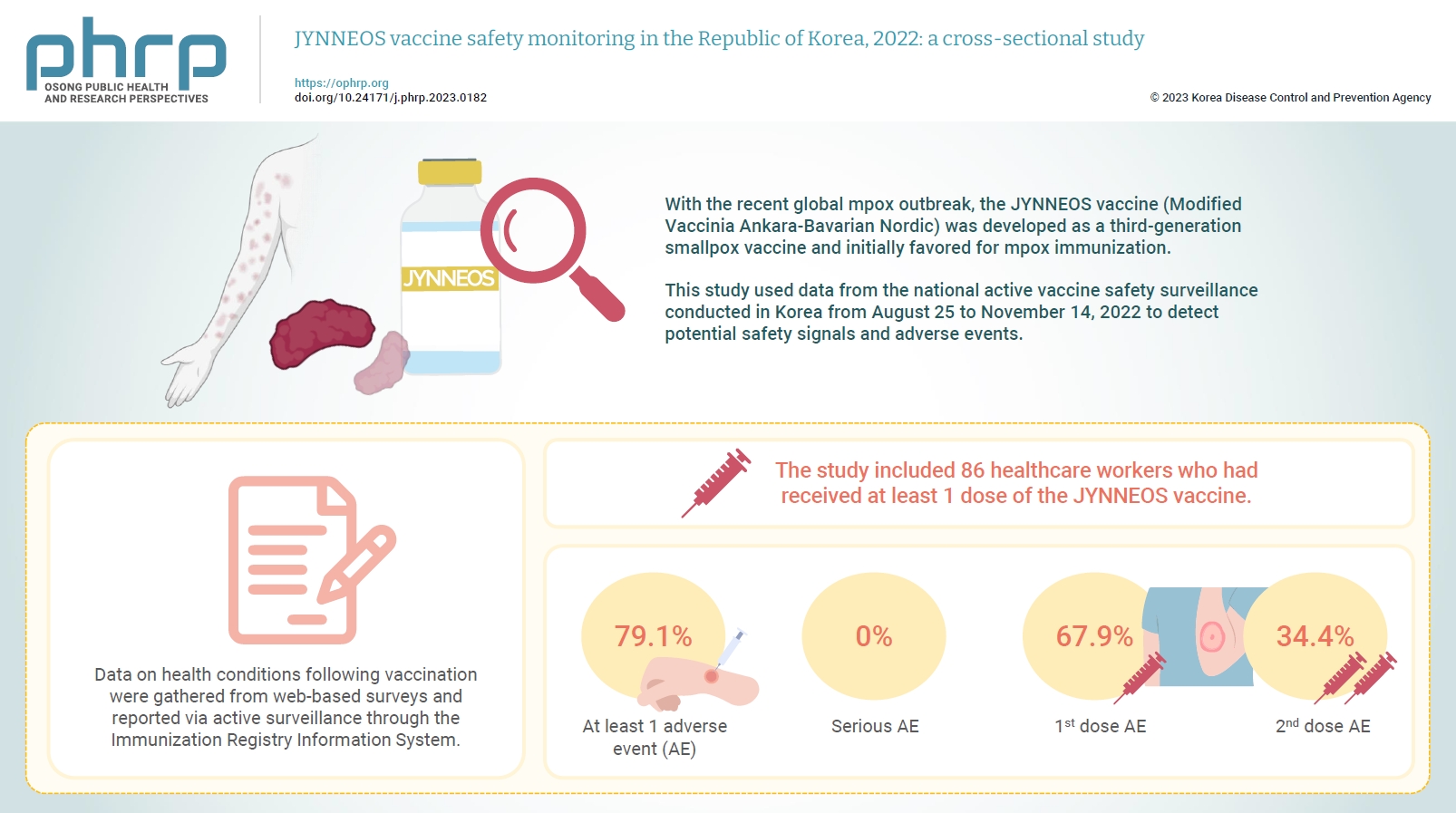
JYNNEOS vaccine safety monitoring in the Republic of Korea, 2022: a cross-sectional study -
Jaeeun Lee1,2
 , Seunghyun Lewis Kwon1,2,3
, Seunghyun Lewis Kwon1,2,3 , Jinhee Park1,2
, Jinhee Park1,2 , Hyuna Bae1,2
, Hyuna Bae1,2 , Hyerim Lee2,4
, Hyerim Lee2,4 , Geun-Yong Kwon2,4
, Geun-Yong Kwon2,4
-
Osong Public Health and Research Perspectives 2023;14(5):433-438.
DOI: https://doi.org/10.24171/j.phrp.2023.0182
Published online: October 18, 2023
1Division of Immunization, Bureau of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
2Central Disease Control Headquarters of Monkeypox, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
3KDI School of Public Policy and Management, Sejong, Republic of Korea
4Division of Immunization Planning, Bureau of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
- Corresponding author: Geun-Yong Kwon Division of Immunization Planning, Bureau of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agency, 200 OsongSaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju 28159, Republic of Korea E-mail: ego1002@korea.kr
• Received: June 30, 2023 • Revised: September 4, 2023 • Accepted: September 8, 2023
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Figure & Data
References
Citations
Citations to this article as recorded by 

- Adverse Reactions After Intradermal Vaccination With JYNNEOS for Mpox in Korea
So Yun Lim, Yu Mi Jung, Yeonjae Kim, Gayeon Kim, Jaehyun Jeon, BumSik Chin, Min-Kyung Kim
Journal of Korean Medical Science.2024;[Epub] CrossRef
- Figure
- Related articles
-
- COVID-19 infection among people with disabilities in 2021 prior to the Omicron-dominant period in the Republic of Korea: a cross-sectional study
- Evaluation of COVID-19 vaccine effectiveness in different high-risk facility types during a period of Delta variant dominance in the Republic of Korea: a cross-sectional study


 Cite
Cite