Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(5); 2023 > Article
-
Original Article
Evaluation of COVID-19 vaccine effectiveness in different high-risk facility types during a period of Delta variant dominance in the Republic of Korea: a cross-sectional study -
Min Jei Lee1
 , Myung-Jae Hwang2
, Myung-Jae Hwang2 , Dong Seob Kim3
, Dong Seob Kim3 , Seon Kyeong Park3
, Seon Kyeong Park3 , Jihyun Choi1
, Jihyun Choi1 , Ji Joo Lee1
, Ji Joo Lee1 , Jong Mu Kim1
, Jong Mu Kim1 , Young-Man Kim1
, Young-Man Kim1 , Young-Joon Park1
, Young-Joon Park1 , Jin Gwack4
, Jin Gwack4 , Sang-Eun Lee1
, Sang-Eun Lee1
-
Osong Public Health and Research Perspectives 2023;14(5):418-426.
DOI: https://doi.org/10.24171/j.phrp.2023.0188
Published online: October 19, 2023
1Central Disease Control Headquarters, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
2Division of Public Health Emergency Response Research, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
3Gyeonam Regional Center for Disease Control and Prevention, Korea Disease Control and Prevention Agency, Busan, Republic of Korea
4Division of Infectious Disease Control, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
- Corresponding author: Sang-Eun Lee Central Disease Control Headquarters, Korea Disease Control and Prevention Agency, 187 OsongSaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju 28159, Republic of Korea E-mail: ondalgl@korea.kr
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 1,275 Views
- 44 Download
- 1 Scopus
Abstract
-
Objectives
- We evaluated the effectiveness of coronavirus disease 2019 vaccination in high-risk facilities in the Republic of Korea during the period when the highly transmissible Delta variant was prevalent. Additionally, we aimed to explore any disparities in vaccine effectiveness (VE) across various types of institutions, specifically distinguishing between non-medical and medical establishments.
-
Methods
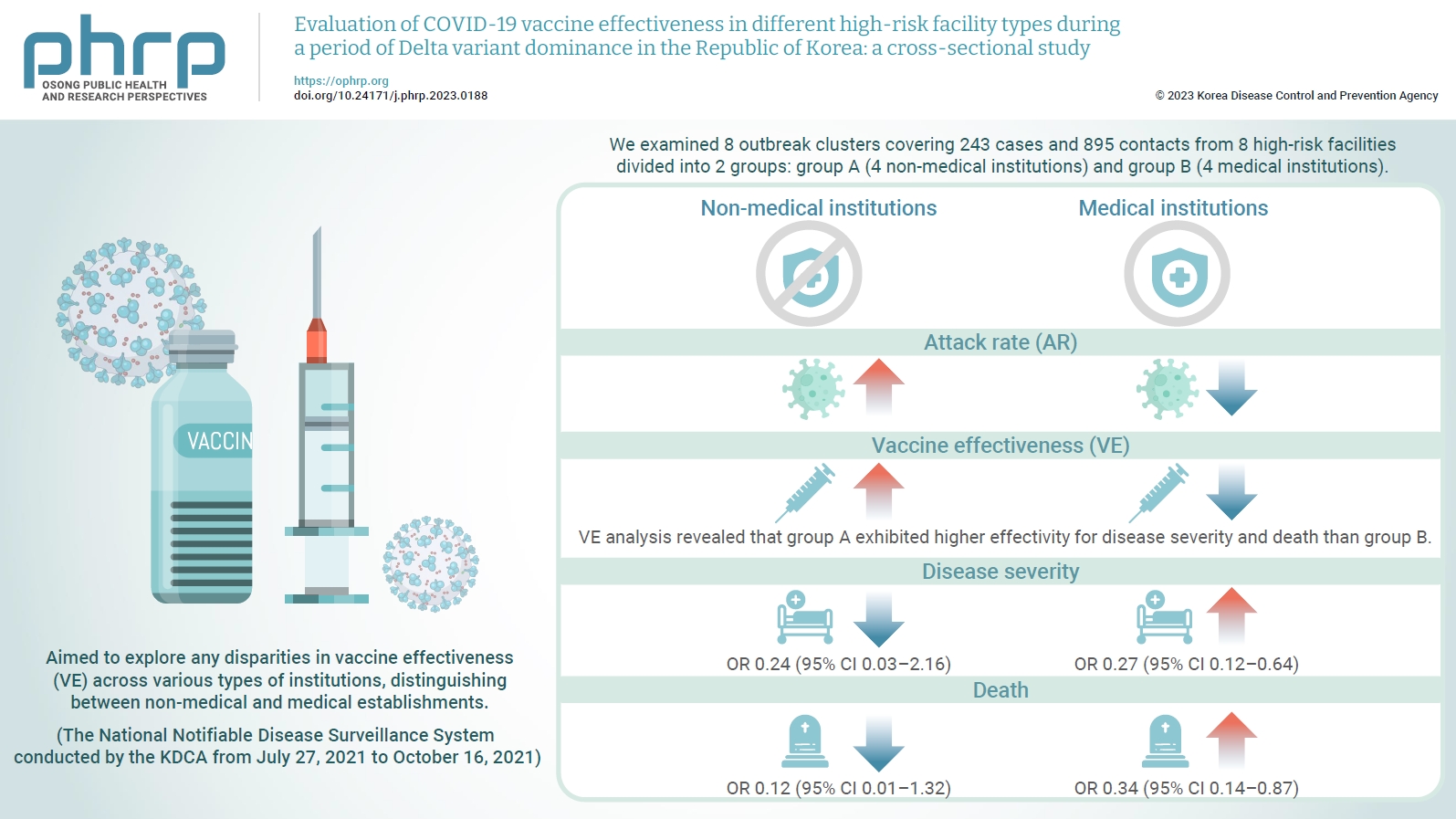
- We examined 8 outbreak clusters covering 243 cases and 895 contacts from 8 high-risk facilities divided into 2 groups: group A (4 non-medical institutions) and group B (4 medical institutions). These clusters were observed from July 27, 2021 to October 16, 2021 for the attack rate (AR) and VE with respect to disease severity. A generalized linear model with a binomial distribution was used to determine the odds ratio (OR) for disease severity and death.
-
Results
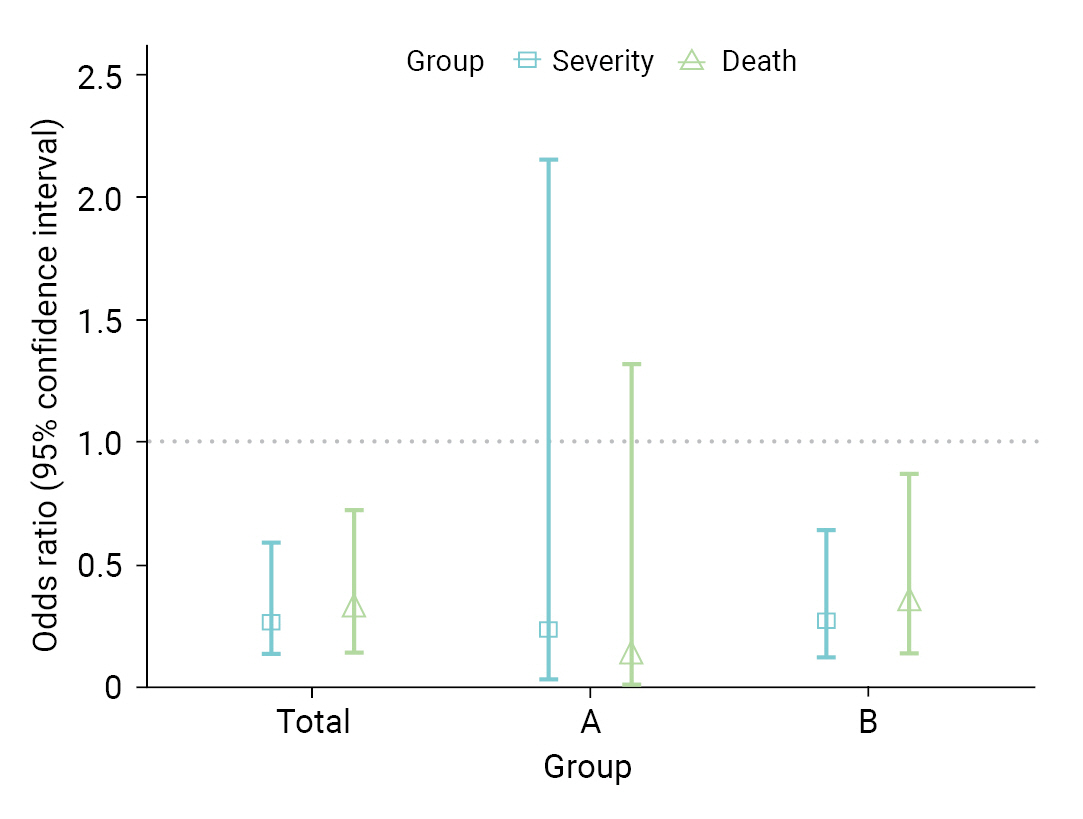
- AR was notably lower in group B (medical institutions). Furthermore, VE analysis revealed that group A exhibited higher effectivity for disease severity and death than group B. The OR for disease severity was 0.24 (95% confidence interval [CI], 0.03–2.16) for group A and 0.27 (95% CI, 0.12–0.64) for group B, with the OR for death at 0.12 (95% CI, 0.01–1.32) in group A and 0.34 (95% CI, 0.14–0.87) in group B.
-
Conclusion
- Although VE may vary across institutions, our findings underscore the importance of implementing vaccinations in high-risk facilities. Customized vaccination programs, tailored response plans, and competent management personnel are essential for effectively addressing and mitigating public health challenges.
- Coronavirus disease 2019 (COVID-19) has become widespread since it was first reported in Wuhan, China in December 2019 [1]. COVID-19 spreads from person to person, mainly through direct contact with infected individuals or exposure to respiratory droplets [2]. Airborne infections are uncommon; however, in special circumstances such as aerosol-generating procedures in medical institutions and environments where droplets are produced for a long time in an enclosed space, the virus can spread beyond the typical 2-m range of respiratory droplets [3]. New variants of COVID-19 have emerged during the pandemic [4]. These variants are classified as those of concern, interest, or monitoring. In particular, B.1.617.2, also known as the Delta variant, was designated as a variant of concern by the World Health Organization on May 11, 2021 [5]. The risk of hospitalization doubled during infection with the Delta variant compared to infection with the Alpha variant [6].
- Previous studies have shown that vaccination is an effective way to reduce the severity and mortality of COVID-19 [7]. On December 8, 2020, the United Kingdom became the first country to implement a COVID-19 vaccination program after emergency authorization [8]. In the Republic of Korea, the national vaccination program began on February 26, 2021, shortly after emergency authorization. Patients and healthcare workers in long-term care facilities and healthcare workers who directly cared for COVID-19 patients were prioritized for vaccination [9]. The preventive effects of COVID-19 vaccines have been demonstrated nationally and internationally [10].
- Nursing institutions for older adults, where vulnerable aging populations with low immunity live in groups, are structurally susceptible to the spread of infectious diseases, which may cause fatalities in cases of severe infections [11]. It is difficult to maintain a safe distance between residents because of their spatial characteristics, as well as because residents and staff carry out activities in confined spaces with a high frequency of physical contact [12]. Therefore, there is a critical need to consistently provide scientific evidence that can be utilized to proactively respond to outbreaks of respiratory infectious diseases such as COVID-19 in high-risk facilities such as nursing homes.
- In this study, we aimed to observe the epidemiological characteristics and vaccine effects of 8 high-risk facilities reported to the Korea Disease Control and Prevention Agency (KDCA) from July 27, 2021, to October 16, 2021 (when the Delta variant predominated), when both the incidence and mortality of COVID-19 increased [13]. In addition, we divided the 8 high-risk facilities into non-medical and medical institutions and evaluated the differences according to institutional characteristics. Based on reliable information from these 8 institutions (older adult day care centers, nursing homes, and nursing hospitals), the goal was to review past measures to prevent infection and reduce severity and death, thereby providing scientific evidence for future public health response strategies.
- Although the Delta variant is no longer the dominant strain, new variants with potential resistance to existing immunity continue to emerge. Notably, as of the fourth week of July 2023, the number of daily confirmed cases has surged by 17% from the previous week, averaging 45,529 cases. This marks the fifth consecutive week of such escalation. In light of these developments, experts are increasingly concerned about the risk of COVID-19 infection in vulnerable populations, especially the elderly and those with underlying medical conditions. They recommend COVID-19 vaccination as a protective measure for these groups [14]. The ongoing emergence of these variants underscores the importance of strict surveillance of these high-risk groups. Our research provides crucial insights for the continued adoption of vaccine policies targeting these groups.
Introduction
- Data Source
- This study was based on confirmed COVID-19 data from the mass outbreak reported to the National Notifiable Disease Surveillance System conducted by the KDCA from July 27, 2021 to October 16, 2021. A confirmed COVID-19 case was defined as a COVID-19 infection confirmed through a real-time reverse transcriptase-polymerase chain reaction test regardless of clinical symptoms. After a cluster outbreak was identified, a study was conducted on patients related to the cluster outbreak in 8 institutions (2 older adult day care centers, 2 nursing homes, and 4 nursing hospitals), from which epidemiological information was collected through field epidemiological investigations. The criteria for enrollment in a cluster were based on 10 or more confirmed cases with epidemiological associations [15]. The data included the number of cases in the order of diagnosis, date of laboratory-confirmed COVID-19 diagnosis, vaccination, sex, and age. To evaluate severity according to vaccination status after case confirmation, we included patients in critical condition requiring high-flow nasal cannula oxygen therapy, mechanical ventilation, or extracorporeal membrane oxygenation; those treated with continuous renal replacement therapy; and those with multiple organ failure, based on information obtained from the Health Insurance Review and Assessment Service System [16]. During the study period, reported deaths were classified as deaths due to COVID-19.
- Study Participants
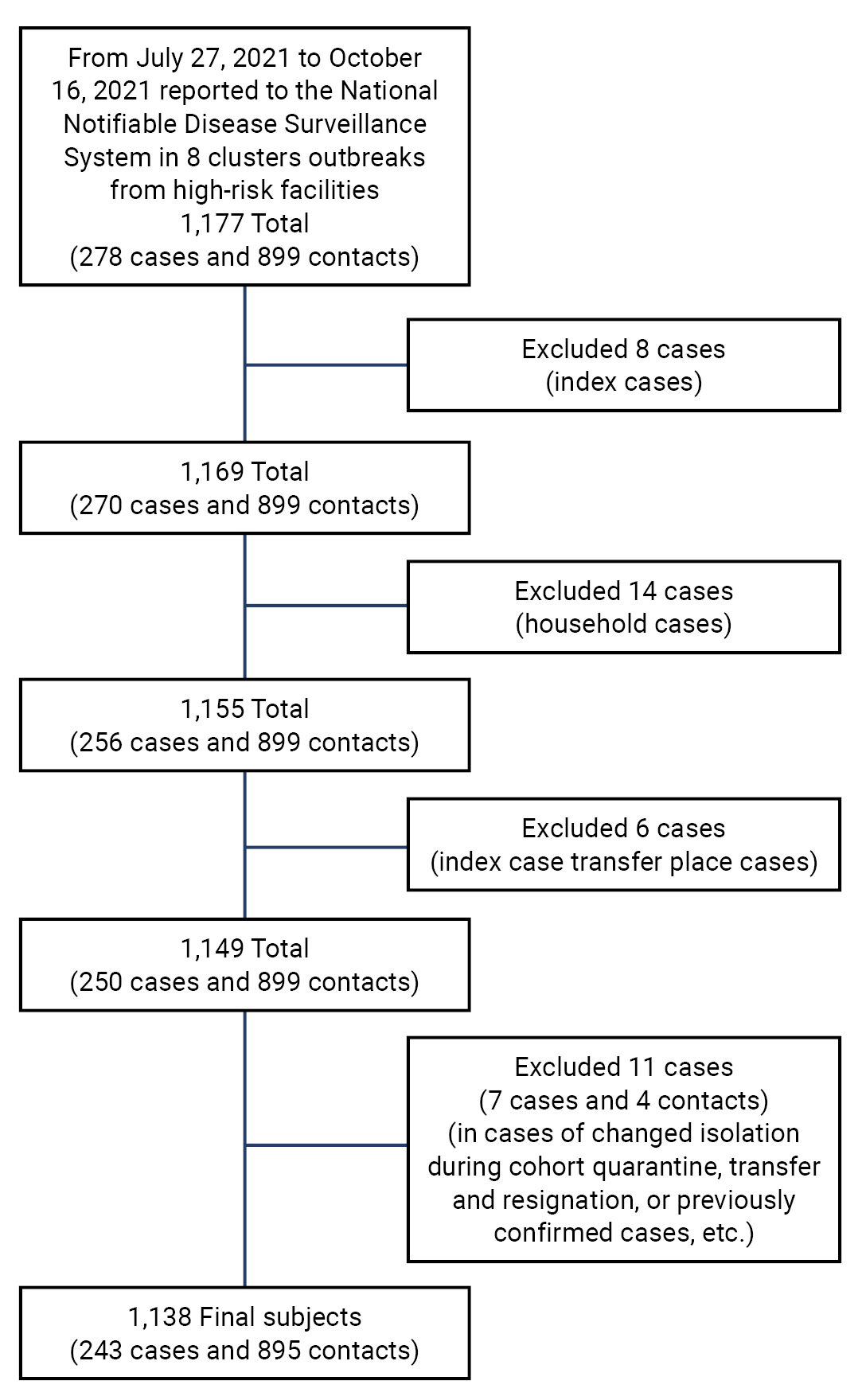
- There were 1,177 cases reported in the 8 high-risk facilities, including 278 confirmed cases related to nursing institutions and 899 contacts. Among them, 8 index cases, 14 confirmed cases with family contact, 6 confirmed cases at the place of index case transfer, and 11 other cases (changes in quarantine location during cohort quarantine, transfer and discharge cases, and previously confirmed cases, among others) were excluded. A total of 1,138 cases (243 confirmed cases and 895 contacts) were included in the study. Contacts other than confirmed cases were defined as those who had been subjected to self-quarantine or cohort quarantine after the occurrence of an index case and were classified as contacts after the exposure scale evaluation (Figure 1).
- Study Design
- Before evaluating vaccine effectiveness (VE), we divided the 8 high-risk facilities for older adults into 2 groups, A and B, corresponding to non-medical and medical facilities, respectively. Group A included 2 older adult day care centers and 2 nursing homes, and group B included 4 nursing hospitals. In this study, data on the history of COVID-19 vaccination in patients confirmed with COVID-19 were obtained from the Immunization Registry Information System. Patients were classified based on their COVID-19 vaccination status as follows: (1) completely vaccinated—14 days or more after completing vaccination; (2) incompletely vaccinated—14 days or less after at least 1 dose of vaccination; and (3) unvaccinated.
- The attack rate (AR) was calculated as the percentage of the number of confirmed cases relative to the number of individuals studied. The severity rate was calculated as the percentage of the number of severe cases and the number of deaths among the confirmed cases. The fatality was calculated as the percentage of the number of deaths relative to the number of confirmed cases during the study period. Severity rates were calculated along with death status, because severity could be underestimated if critical patients died before reaching an intensive care unit, such as a nursing hospital, meaning that some severe patients would be excluded from consideration [17]. After evaluating the effect of vaccination on disease severity and fatality in the incompletely vaccinated (including unvaccinated) and completely vaccinated groups, the odds ratio (OR) was calculated.
- Statistical Analysis
- To analyze the general characteristics of the 1,138 cases in this study, we divided the confirmed and non-confirmed cases (contacts) into 2 groups: A and B. The 243 confirmed cases were categorized by sex, age, status (resident or worker), and vaccination history, and were additionally categorized by severity (severe or death). The chi-square and Fisher's exact tests were used to determine differences in group composition. To evaluate confirmed COVID-19 cases, we calculated the AR as follows: AR=[(number of people with the disease)/(number of people at risk)]×100.
- To evaluate the effectiveness of vaccination against COVID-19 severity and fatality, we calculated the VE as follows: VE=[1–(completed vaccination %/unvaccinated or incomplete vaccination %)]×100.
- Additionally, to evaluate the effect of vaccination on the severity and death of COVID-19, we used generalized linear models (GLMs) with binomial distributions. Emphasizing the effect of vaccination, the results were displayed as ORs with 95% confidence intervals (CIs). We performed analyses of the overall study population and separately within the 2 institutional groups (A and B), enabling a comparison across different settings. R statistical software ver. 4.2.2 (The R Foundation) was used, and statistical significance was set at p=0.05.
- Ethics Approval
- This study was approved by the Institutional Review Board (IRB) of the KDCA (IRB No: KDCA 2021-12–02-PE-A). The Board waived the requirement for informed consent.
Materials and Methods
- General Characteristics
- Table 1 shows the general characteristics of the 8 high-risk facilities, including their vaccination histories. The facilities included 2 day care centers for older adults and 2 nursing homes in group A, and 4 nursing hospitals in group B. A total of 1,138 patients were included, with 137 in group A and 1,001 in group B. The proportion of confirmed patients in group A was 48.2%, whereas the proportion was 17.7% in group B (p<0.005). Among the confirmed cases, most patients were aged ≥65 years (81.8% and 79.7% in groups A and B, respectively). Additionally, among the confirmed cases, residents (patients and older adults) accounted for the majority of the cases (77.3% and 81.9% in groups A and B, respectively). A total of 35 severe cases (4 in group A and 31 in group B) were observed. There were 3 deaths (75.0%) in group A and 26 deaths (83.9%) in group B.
- AR, Severity Rate, and Fatality in Eight High-Risk Facilities
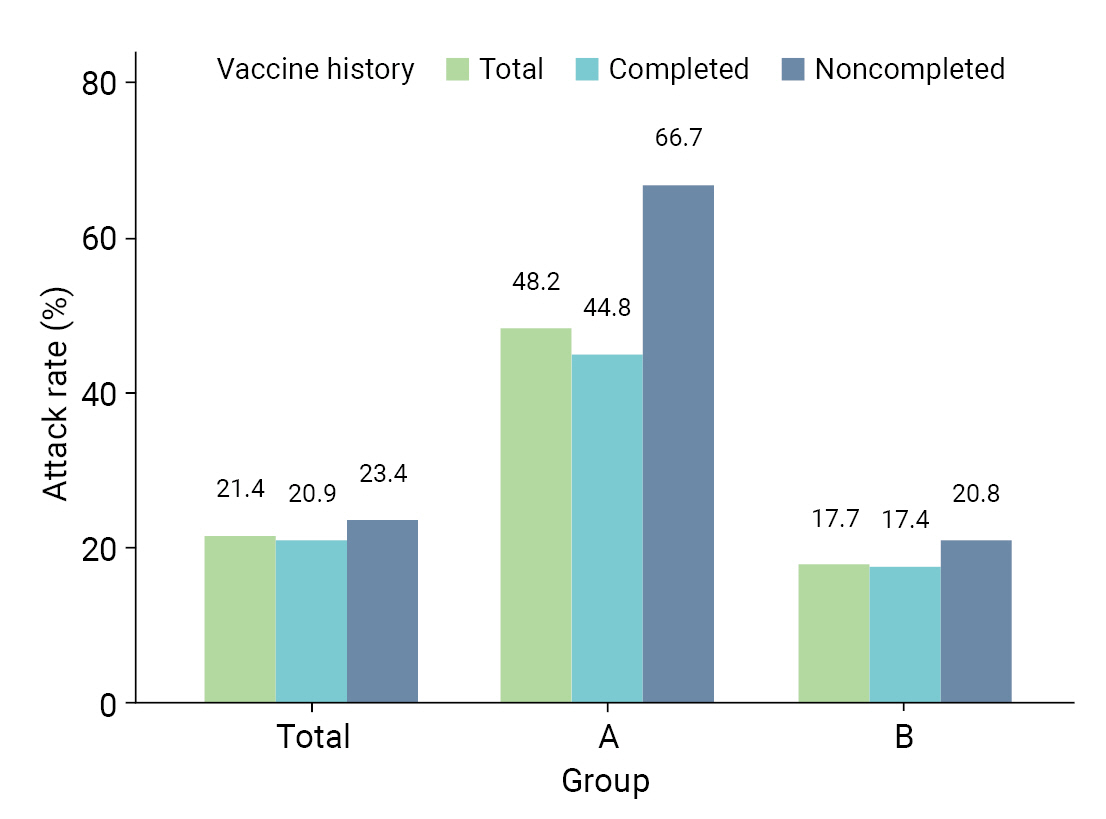
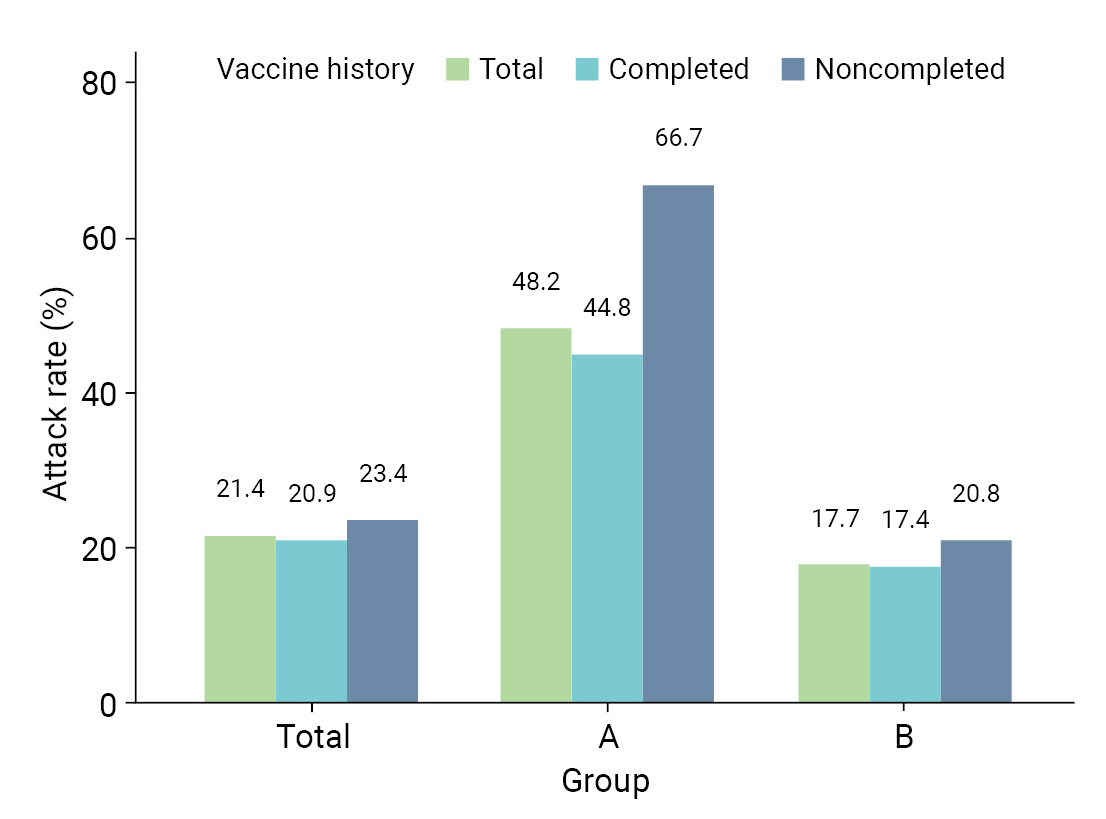
- Table 2 provides a comparative analysis of AR, severity, and fatality in the 8 high-risk facilities. AR was higher in group A than in group B; specifically, AR was higher in both males (47.4% vs. 17.8%) and females (48.5% vs. 17.6%). In group A, the ARs were higher in both age groups, 32.4% for those aged <65 years and 54.0% for those aged ≥65 years, which were both higher than the corresponding rates of 9.5% and 22.7% in group B. Moreover, the AR among residents, which included patients and older adults, was higher in group A (55.4%) than in group B (23.1%). Similarly, workers in group A experienced a higher AR (33.3%) than those in group B (8.6%). For vaccination history, the AR was higher in group A for unvaccinated patients (62.5% vs. 17.6%), followed by patients with incomplete vaccination (80.0% vs. 20.5%) and complete vaccination (44.8% vs. 17.4%) (Figure 2). The severity rate was lower in group A than in group B for both the total population and those aged ≥65 years. However, the severity rate was higher in both groups in those who were not vaccinated or had incomplete vaccinations. The fatality was lower in group A than in group B for both the total population and those aged ≥65 years. However, the fatality was higher in both groups in those who were not vaccinated or had incomplete vaccinations (Table 2).
- Vaccination Effectiveness
- Table 3 shows a comparison of VE in the 8 high-risk facilities. In group A, the VE for severity and death were 73.1% and 86.5%, respectively. In contrast, in group B, VE was 63.4% for severity and 57.7% for fatality. In total, the VE was 64.6% for severity (95% CI, 35.9%–80.4%) and 62.4% for death (95% CI, 26.4%–80.8%). As a result of the GLM, the OR for severe disease among vaccinated individuals was 0.27 (95% CI, 0.13–0.60); whereas for death, it was 0.31 (95% CI, 0.14–0.73) (Figure 3). When analyses were performed separately within the institutional groups, group A showed an OR of 0.24 (95% CI, 0.03–2.16) for severity and 0.12 (95% CI, 0.01–1.32) for death. However, these results were not statistically significant. Conversely, in group B, the OR for severity was 0.27 (95% CI, 0.12–0.64); whereas for death, it was 0.34 (95% CI, 0.14–0.87).
Results
- Comparison of Confirmed Cases in Non-Medical and Medical Institutions
- This study provides crucial epidemiological insights into the transmission dynamics of COVID-19 and the effectiveness of vaccination in high-risk facilities. A significant observation was the higher rate of confirmed cases in non-medical institutions (group A) than in medical institutions (group B). This disparity suggests potential structural or operational risk factors unique to group A, which is consistent with previous studies demonstrating that an increase in medical staff is associated with a decrease in infection risk [18,19]. Our study revealed that a considerable proportion of confirmed cases in both groups involved individuals aged ≥65 years, reflecting the increased COVID-19 susceptibility in older individuals. Moreover, residents (including patients and older adults) comprised the majority of patients with confirmed cases, suggesting an elevated risk due to their living arrangements and potentially compromised immunity. This observation is consistent with that of a Spanish study that reported a higher infection rate among nursing home residents than among staff [20]. In terms of case severity, our findings indicate that baseline conditions in both healthcare and non-healthcare settings could potentially influence patient outcomes [21]. These findings highlight the necessity of considering these conditions when developing future infection control strategies for high-risk environments.
- Comparison of AR, Severity, and Fatality according to Facility Type
- We analyzed the ARs and severity of COVID-19 to provide crucial information for assessing the situation in high-risk facilities. We found that group A (non-medical institutions) had a higher overall AR than group B (medical institutions). This discrepancy was more pronounced when age and vaccination history were considered. For individuals aged ≥65 years, group A had an AR of 54.0%, which was significantly higher than that in group B (22.7%). Group A also had higher ARs among individuals who were unvaccinated, partially vaccinated, or fully vaccinated. In both groups, the AR was higher among unvaccinated and partially vaccinated individuals than among fully vaccinated individuals. Furthermore, the elevated severity rate and fatality among unvaccinated and partially vaccinated individuals underscore the importance of vaccination. Our findings are consistent with those of previous research on the Delta variant surge in India, confirming the effectiveness of vaccines as a protective measure against COVID-19 [22]. These findings suggest the need for strategies to improve vaccination rates in high-risk facilities.
- Comparison of COVID-19 VE and Risk Level according to Facility Type
- The severity and fatality rates in the complete vaccination group were 10.4% and 8.9%, respectively. Additionally, in the comparison of VE in high-risk facilities, significant disparities were observed between group A (day care centers and nursing homes for older adults) and group B (nursing hospitals) in terms of preventing severe disease and fatality. Group A demonstrated an effectiveness rate of 73.1% for preventing severe disease and 86.5% for preventing fatalities. In contrast, group B showed an effectiveness rate of 63.4% for preventing severe disease and 57.7% for preventing fatalities. These findings align with those of previous studies conducted in the United Kingdom [23] and further emphasize the strong protective effects of COVID-19 vaccination among older adults residing in day care centers and long-term care facilities. The differences in VE between groups A and B may have been influenced by various factors, including population characteristics, infection control measures, and healthcare resources specific to each setting [24]. Further GLM analysis further confirmed the consistency of the results with the initial VE assessment. However, it was not statistically significant for group A, which appears to be a result of widened CIs due to the limited sample size. However, it is noteworthy that significant OR results were reported in another study investigating COVID-19 outbreaks in nursing facilities located in Gwangju [25]. Considering these findings, the preventive effect of COVID-19 vaccination should be considered in future follow-up studies. This comprehensive analysis provides valuable insights into the efficacy of vaccination in high-risk facilities and emphasizes the importance of tailored strategies to maximize protection in these settings.
- Limitation and Strengths
- Our study has several limitations. First, our analysis primarily focused on older individuals in high-density settings and generalization of the results to the entire population may be limited. It is crucial to continuously monitor and study groups that can contribute to increased transmission and group infections, such as schools, crowded public spaces, and workplaces. This consideration extends beyond the scope of COVID-19 to encompass various infectious diseases. However, comparing the effectiveness of interventions and preventive measures between medical and non-medical institutions in high-risk facilities for older individuals is particularly valuable. This comparative analysis allowed a deeper understanding of the specific challenges and factors influencing the outcomes in each setting. By identifying similarities and differences between different settings, interventions and strategies can be tailored to effectively mitigate the risk of infection and optimize health outcomes in older populations. Second, we conducted a study based on information obtained during the Delta-predominant period. In Republic of Korea, the peak period of Delta predominance has passed. Even though the peak period of Delta predominance has passed, research related to COVID-19 vaccines continues globally. Our evaluation of VE in high-risk older adults is consistent with recent findings that emphasize the need for transparent information about the effectiveness and reliability of vaccination in older adults [26]. In addition, recent studies of vaccine hesitancy have highlighted the importance of consensus building using relevant evidence from healthcare professionals and scientists [27] and the potential for interventions [28]. This study is important to the extent that it holds meaning in providing evidence related to VE in high-risk populations within a controlled setting, both nationally and internationally. Such evidence could be helpful in advancing health policy related to vaccination, and continuation of research targeting these high-risk groups is imperative.
- Additionally, we conducted this study using information directly collected by central epidemiological investigators and local public health response personnel after the announcement of the COVID-19 vaccination program for older adults aged ≥65 years residing in medical and care facilities by the Korean government on March 23, 2021 [29]. In particular, we collected cluster information during the Delta-predominant period, which had a high fatality rate [30,31], to provide insights into how to manage the spread and prevalence of new COVID-19 variants. Furthermore, we analyzed the differences in the effectiveness of the preventive vaccine between the completely and incompletely vaccinated groups (including the unvaccinated group). We analyzed data from 8 medical and care facilities for older adults, a high-risk group in densely populated environments for which vaccination was prioritized. This evaluation of the effectiveness of public health response measures may be valuable in establishing response strategies in the event of future novel respiratory infections.
- Implications for VE and Infection Control Policies
- This study aimed to examine the effectiveness of preventive vaccines based on differences in communal facilities among high-risk COVID-19 management groups. This study confirmed that vaccination policies in medical facilities for older adults (day care centers, nursing homes, and nursing hospitals) could effectively reduce the risk of severe illness and death during the Delta-predominant period. Furthermore, as highlighted in this study, the appropriate establishment and use of vaccination in conjunction with infection control recommendations could be effective for the continuously emerging COVID-19 variants and new respiratory infections [32]. In addition, well-trained management personnel are recommended. Finally, future case studies based on field epidemiological investigations, such as this study, to analyze the effectiveness of infection control measures according to medical personnel standards in infection-prone facilities and obtain information from nursing hospitals during the COVID-19 outbreak can support the establishment of scientific evidence to formulate future infection control policies [33].
Discussion
- There were significant differences between the 2 institutional types. Our study demonstrated the effectiveness of COVID-19 vaccines in preventing severe disease and deaths in high-risk communal facilities. These findings highlight the importance of vaccination as a crucial strategy for managing respiratory infections including COVID-19. These results contribute to the existing literature and emphasize the need for further research to establish effective infection prevention measures. Developing unique response plans for each facility type and ensuring the presence of well-trained management personnel are critical. Through continual research and adjustments to strategies, we can optimize the protection of vulnerable populations and enhance public health outcomes.
Conclusion
- • Vaccination significantly reduced disease severity and death in high-risk facilities during a period of Delta variant dominance.
- • Variations in vaccine effectiveness were observed between non-medical and medical institutions.
- • Customized vaccination programs and response plans are essential for protecting vulnerable populations and improving public health outcomes in high-risk facilities.
HIGHLIGHTS
-
Ethics Approval
This study was approved by the IRB of the KDCA (IRB No: KDCA 2021-12–02-PE-A). The Board waived the requirement for informed consent.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
These data sets are not publicly available. If you have any questions regarding this study, contact the corresponding author (ondalgl@korea.kr).
-
Authors’ Contributions
Conceptualization: MJL, YJP; Data curation: DSK, SKP, JC, JJL, JMK, YMK; Formal analysis: MJL; Methodology: MJL, YJP; Project administration: JG, SEL; Visualization: MJL, MJH; Writing–original draft: MJL; Writing–review and editing: all authors. All authors read and approved the final manuscript.
Article information
-
Acknowledgements
- The authors thank the public health response agents from each region for making this study possible.
| Characteristic |
8 High-risk facilities |
pa) | ||
|---|---|---|---|---|
| Group A | Group B | Total | ||
| Total | 137 (100.0) | 1,001 (100.0) | 1,138 (100.0) | |
| Status | <0.005 | |||
| Confirmed | 66 (48.2) | 177 (17.7) | 243 (21.4) | |
| Non-confirmed | 71 (51.8) | 824 (82.3) | 895 (78.6) | |
| Confirmed case | ||||
| Sex | 1.000 | |||
| Male | 18 (27.3) | 47 (26.6) | 65 (26.7) | |
| Female | 48 (72.7) | 130 (73.4) | 178 (73.3) | |
| Age (y) | 0.846 | |||
| <65 | 12 (18.2) | 36 (20.3) | 48 (19.8) | |
| ≥65 | 54 (81.8) | 141 (79.7) | 195 (80.2) | |
| Status | 0.527 | |||
| Residents (patients and older adults)b) | 51 (77.3) | 145 (81.9) | 196 (80.7) | |
| Worker | 15 (22.7) | 32 (18.1) | 47 (19.3) | |
| Vaccine history | <0.005 | |||
| Unvaccinated | 10 (15.2) | 21 (11.9) | 31 (12.8) | |
| Incomplete vaccination | 4 (6.1) | 16 (9.0) | 20 (8.2) | |
| Complete vaccination | 52 (78.8) | 140 (79.1) | 192 (79.0) | |
| Case severity | ||||
| Severity | ||||
| Total | 4 (100.0) | 31 (100.0) | 35 (100.0) | |
| Severec) | 1 (25.0) | 5 (16.1) | 6 (17.1) | 0.546 |
| Death | 3 (75.0) | 26 (83.9) | 29 (82.9) | |
Group A comprised 2 day care centers for older adults and 2 nursing homes, while group B comprised 4 nursing hospitals.
a) p-values were obtained by comparing the groups using the chi-square or Fisher's exact tests.
b) The term “resident” refers to patients and older adults in day care centers for older adults, nursing homes, and nursing hospitals.
c) Severe was defined as excluding death.
| Variable |
8 High-risk facilities |
pa) | ||
|---|---|---|---|---|
| Group A | Group B | Total | ||
| Total | 48.2 (40.0–56.5) | 17.7 (15.4–20.1) | 21.4 (19.1–23.8) | |
| Sex | 1.000 | |||
| Male | 47.4 (32.5–62.7) | 17.8 (13.7–22.9) | 21.5 (17.3–26.5) | |
| Female | 48.5 (38.9–58.2) | 17.6 (15.1–20.6) | 21.3 (18.7–24.2) | |
| Age (y) | 0.547 | |||
| <65 | 32.4 (19.6–48.5) | 9.5 (6.9–12.8) | 11.5 (8.8–14.9) | |
| ≥65 | 54.0 (44.3–63.4) | 22.7 (19.6–26.2) | 27.0 (23.9–30.4) | |
| Status | 0.399 | |||
| Residents (patients and older adults)b) | 55.4 (45.3–65.2) | 23.1 (20.0–26.5) | 27.2 (24.1–30.6) | |
| Worker | 33.3 (21.4–47.9) | 8.6 (19.6–26.2) | 11.2 (8.6–14.6) | |
| Vaccine history | 0.523 | |||
| Unvaccinated | 62.5 (38.6–81.5) | 17.6 (11.8–25.5) | 23.0 (16.7–30.7) | |
| Incomplete vaccination | 80.0 (37.6–96.4) | 20.5 (13.0–30.8) | 24.1 (16.2–34.3) | |
| Complete vaccination | 44.8 (36.1–53.4) | 17.4 (14.9–20.2) | 20.9 (18.4–23.6) | |
| Severity and fatality ratesc) | ||||
| Severity | ||||
| Total | 6.1 (2.4–14.6) | 17.5 (12.6–23.8) | 14.4 (10.5–19.4) | |
| Age (y) | 1.000 | |||
| <65 | 0 | 2.8 (0.5–14.2) | 2.1 (0.4–10.9) | |
| ≥65 | 7.4 (2.9–17.6) | 21.3 (15.3–28.7) | 17.4 (12.8–23.4) | |
| Vaccine history | 0.763 | |||
| Unvaccinated incomplete vaccination | 14.3 (4.0–39.9) | 35.1 (21.8–51.2) | 29.4 (18.7–43.0) | |
| Complete vaccination | 3.8 (1.1–13.0) | 12.9 (8.3–19.4) | 10.4 (6.8–15.5) | |
| Death | ||||
| Total | 4.5 (1.6–12.5) | 14.7 (10.2–20.7) | 11.9 (8.4–16.6) | |
| Age (y) | 1.000 | |||
| <65 | 0 | 2.8 (4.9–14.2) | 2.1 (0.4–10.9) | |
| ≥65 | 5.6 (1.9–15.1) | 17.7 (12.3–24.9) | 14.4 (10.1–20.0) | |
| Vaccine history | 0.301 | |||
| Unvaccinated incomplete vaccination | 14.3 (4.0–39.9) | 27.0 (15.4–43.0) | 23.5 (14.0–36.8) | |
| Complete vaccination | 1.9 (0.3–10.1) | 11.4 (7.2–17.8) | 8.9 (5.6–13.7) | |
Data are presented as attack rate (95% confidence interval). 95% confidence intervals were calculated using the Wilson score interval method. Group A comprised 2 day care centers for older adults and 2 nursing homes, while group B comprised 4 nursing hospitals.
a) p-values were obtained by comparing the groups using the chi-square or Fisher exact tests.
b) The term “resident” refers to patients and older adults in day care centers for older adults, nursing homes, and nursing hospitals.
c) The severity rate was calculated as the percentage of the number of severe cases and the number of deaths among the confirmed cases, and the fatality rate was calculated as the percentage of deaths among confirmed cases during the study period.
| Variable | 8 High-risk facilities | ||
|---|---|---|---|
| Group A | Group B | Total | |
| Severityb) | 73.1 (–74.5 to 95.8) | 63.4 (32.3 to 80.2) | 64.6 (35.9 to 80.4) |
| Deathb) | 86.5 (–37.9 to 98.7) | 57.7 (14.7 to 79.0) | 62.4 (26.4 to 80.8) |
Data are presented as vaccine effectiveness (95% confidence interval). 95% confidence intervals were calculated using the Wilson score interval method. Group A comprised 2 day care centers for older adults and 2 nursing homes, while group B comprised 4 nursing hospitals.
a) [1–(complete vaccination %/unvaccinated or incomplete vaccination %)]×100.
b) The severity rate was calculated as the percentage of the number of severe cases and the number of deaths among the confirmed cases, and the fatality rate was calculated as the percentage of deaths among confirmed cases during the study period.
- 1. Zhu H, Wei L, Niu P. The novel coronavirus outbreak in Wuhan, China. Glob Health Res Policy 2020;5:6. ArticlePubMedPMCPDF
- 2. Sharma A, Tiwari S, Deb MK, et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents 2020;56:106054. ArticlePubMedPMC
- 3. Korea Disease Control and Prevention Agency (KDCA). Guidelines for response to coronavirus disease-19 (for local government) [Internet]. 13-1st ed. KDCA; 2022 [cited 2023 Sep 28]. Available from:x https://ncov.kdca.go.kr/shBoardView.do?brdId=2&brdGubun=28&ncvContSeq=6814. Korean.
- 4. Islam S, Islam T, Islam MR. New coronavirus variants are creating more challenges to global healthcare system: a brief report on the current knowledge. Clin Pathol 2022;15:2632010X221075584.ArticlePubMedPMCPDF
- 5. Choi JY, Smith DM. SARS-CoV-2 variants of concern. Yonsei Med J 2021;62:961−8.ArticlePubMedPMCPDF
- 6. Shiehzadegan S, Alaghemand N, Fox M, et al. Analysis of the delta variant B.1.617.2 COVID-19. Clin Pract 2021;11:778−84.ArticlePubMedPMC
- 7. Kim JA, Kim YY, Kim RK, et al. COVID-19 vaccine during May-July 2021 effects of severe and mortality prevention, Republic of Korea [Internet]. Korea Disease Control and Prevention Agency; 2021 [cited 2023 May 2]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=716913&act=viev#. Korean.
- 8. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373:n1088. PubMed
- 9. Nham E, Song JY, Noh JY, et al. COVID-19 vaccination in Korea: past, present, and the way forward. J Korean Med Sci 2022;37:e351.ArticlePubMedPMCPDF
- 10. Choi WS. Comprehensive understanding and field application of COVID-19 vaccine. Korean J Med 2021;96:155−9. Korean.ArticlePDF
- 11. Shon CW, Yoon MS, Kim SA, et al. A study on the COVID-19 outbreak in long-term care facilities of Seoul and the related issue. The Seoul Institute;; 2021. Korean.
- 12. Choi HJ. Causes and counter-measures for the COVID-19 outbreak in the nursing institutions for the older adults [Internet]. Citizens’ Coalition for Economic Justice; 2021 [cited 2023 May 3]. Available from: http://ccej.or.kr/67179. Korean.
- 13. Jang J, Park SY, Ahn SH, et al. One-year report of COVID-19 outbreak in the Republic of Korea, January-December 2021. Public Health Wkly Rep 2022;15:225−34. Korean.
- 14. Cho JY. COVID-19 Pandemic “To protect older adults...”. TBS [Internet]. 2023 Aug 1 [cited 2023 Aug 11]. Available from: http://tbs.seoul.kr/news/newsView.do?typ_800=6&idx_800=3503088&seq_800=20495766. Korean.
- 15. Korea Disease Control and Prevention Agency (KDCA). Guidelines for response to coronavirus disease-19 (for local government) 10-1st ed. KDCA; 2021. Korean.
- 16. Choi JH, Lee MJ, Lee SE, et al. Epidemiological characteristics of COVID-19 outbreaks occurring in 6 long-term care facilities after July 2021, Republic of Korea. Public Health Wkly Rep 2021;14:2621−8. Korean.
- 17. Ryu B, Shin E, Kim NY, et al. Severity of COVID-19 associated with SARS-CoV-2 variants circulating in the Republic of Korea. Public Health Wkly Rep 2022;15:2873−5. Korean, English.Article
- 18. Mitchell BG, Gardner A, Stone PW, et al. Hospital staffing and health care-associated infections: a systematic review of the literature. Jt Comm J Qual Patient Saf 2018;44:613−22.ArticlePubMed
- 19. Dykgraaf SH, Matenge S, Desborough J, et al. Protecting nursing homes and long-term care facilities from COVID-19: a rapid review of international evidence. J Am Med Dir Assoc 2021;22:1969−88.ArticlePubMedPMC
- 20. Escribano P, Perez-Granda MJ, Alonso R, et al. High incidence of COVID-19 at nursing homes in Madrid, Spain, despite preventive measures. Rev Esp Quimioter 2022;35:288−92.ArticlePubMedPMC
- 21. Angelsitter. Comparison of differences in nursing homes, nursing hospitals, day care centers, and silver towns [Internet]. Angelsitter; 2023 [cited 2023 May 14]. Available from: https://angelsitter.co.kr/contents.php?cname=welfare_compare. Korean.
- 22. Thiruvengadam R, Awasthi A, Medigeshi G, et al. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: a test-negative, case-control study and a mechanistic study of post-vaccination immune responses. Lancet Infect Dis 2022;22:473−82.ArticlePubMed
- 23. Paranthaman K, Subbarao S, Andrews N, et al. Effectiveness of BNT162b2 and ChAdOx-1 vaccines in residents of long-term care facilities in England using a time-varying proportional hazards model. Age Ageing 2022;51:afac115. ArticlePubMedPMCPDF
- 24. Cho TY. Korean Association of Nursing Hospitals, “Nursing hospitals and nursing homes are different institutions”. Sunday Seoul [Internet]. 2020 Jun 5 [cited 2023 Feb 1]. Available from: https://www.ilyoseoul.co.kr/news/articleView.html?idxno=396337. Korean.
- 25. Ryu SY, Cho JH, Lee R, et al. Effect of COVID-19 vaccinations on deaths of the COVID-19 cases in some elderly long-term care facilities, Gwangju. J Agric Med Community Health 2022;47:109−20. Korean.
- 26. Tzenios N, Chahine M, Tazanios M. Better strategies for coronavirus (COVID-19) vaccination. Spec J Med Acad Life Sci [Internet]. 2023 [cited 2023 Sep 28];1. Available from: https://doi.org/10.58676/sjmas.v1i2.11.
- 27. Stamm TA, Partheymuller J, Mosor E, et al. Determinants of COVID-19 vaccine fatigue. Nat Med 2023;29:1164−71.ArticlePubMedPMCPDF
- 28. Terrell R, Alami A, Krewski D. Interventions for COVID-19 vaccine hesitancy: a systematic review and narrative synthesis. Int J Environ Res Public Health 2023;20:6082. ArticlePubMedPMC
- 29. Korea Disease Control and Prevention Agency (KDCA). COVID-19 vaccinations for senior citizens aged 65 or older in nursing hospitals and facilities will begin on March 23rd! (Vaccine Briefing, 3.22) [Internet]. KDCA; 2021 [cited 2023 Jan 11]. Available from: https://www.kdca.go.kr/gallery.es?mid=a20503030000&bid=0004&b_list=9&act=view&list_no=114503&nPage=18&vlist_no_npage=32&keyField=&keyWord=&orderby=. Korean.
- 30. Liu J, Wei H, He D. Differences in case-fatality-rate of emerging SARS-CoV-2 variants. Public Health Pract (Oxf) 2023;5:100350. ArticlePubMed
- 31. Kim K, Cho K, Song J, et al. The case fatality rate of COVID-19 during the delta and the omicron epidemic phase: a meta-analysis. J Med Virol 2023;95:e28522.ArticlePubMedPDF
- 32. Korea Disease Control and Prevention Agency (KDCA). With COVID-19 infection control recommendations for medical institutions [Internet]. KDCA; 2022 [cited 2023 Feb 1]. Available from: https://www.kdca.go.kr/filepath/boardSyview.es?bid=0019&list_no=721304&seq=1. Korean.
- 33. Suh EK, Kim HR. Patient care experiences of long-term care hospitals nurses during the COVID-19 pandemic: a phenomenological study. J Korean Gerontol Nurs 2022;24:441−53.ArticlePDF
References
Figure & Data
References
Citations



 Cite
Cite