Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(4); 2023 > Article
-
Original Article
Household secondary attack rates and risk factors during periods of SARS-CoV-2 Delta and Omicron variant predominance in the Republic of Korea -
Jin Lee1
 , Mijeong Ko2
, Mijeong Ko2 , Seontae Kim1
, Seontae Kim1 , Dosang Lim3
, Dosang Lim3 , Gemma Park4
, Gemma Park4 , Sang-Eun Lee1
, Sang-Eun Lee1
-
Osong Public Health and Research Perspectives 2023;14(4):263-271.
DOI: https://doi.org/10.24171/j.phrp.2023.0133
Published online: August 11, 2023
1Central Disease Control Headquarters, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
2Capital Regional Center for Disease Control and Prevention, Korea Disease Control and Prevention Agency, Seoul, Republic of Korea
3Bureau of Chronic Disease Prevention and Control, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
4Chungcheong Regional Center for Disease Control and Prevention, Korea Disease Control and Prevention Agency, Daejeon, Republic of Korea
- Corresponding author: Sang-Eun Lee Central Disease Control Headquarters, Korea Disease Control and Prevention Agency, 187 Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju 28159, Republic of Korea E-mail: ondalgl@korea.kr
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 1,891 Views
- 133 Download
Abstract
-
Objectives
- The household secondary attack rate (SAR) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an important indicator for community transmission. This study aimed to characterize transmission by comparing household SARs and identifying risk factors during the periods of Delta and Omicron variant predominance in Republic of Korea.
-
Methods
- We defined the period of Delta variant predominance (Delta period) as July 25, 2021 to January 15, 2022, and the period of Omicron variant predominance (Omicron period) as February 7 to September 3, 2022. The number of index cases included was 214,229 for the Delta period and 5,521,393 for the Omicron period. To identify the household SARs and risk factors for each period, logistic regression was performed to determine the adjusted odds ratio (aOR).
-
Results
- The SAR was 35.2% for the Delta period and 43.1% for the Omicron period. The aOR of infection was higher in 2 groups, those aged 0 to 18 years and ≥75 years, compared to those aged 19 to 49 years. Unvaccinated individuals (vs. vaccinated individuals) and individuals experiencing initial infection (vs. individuals experiencing a second or third infection) had an increased risk of infection with SARS-CoV-2.
-
Conclusion
- This study analyzed the household SARs and risk factors. We hope that the results can help develop age-specific immunization plans and responses to reduce the SAR in preparation for emerging infectious diseases or potential new variants of SARS-CoV-2.
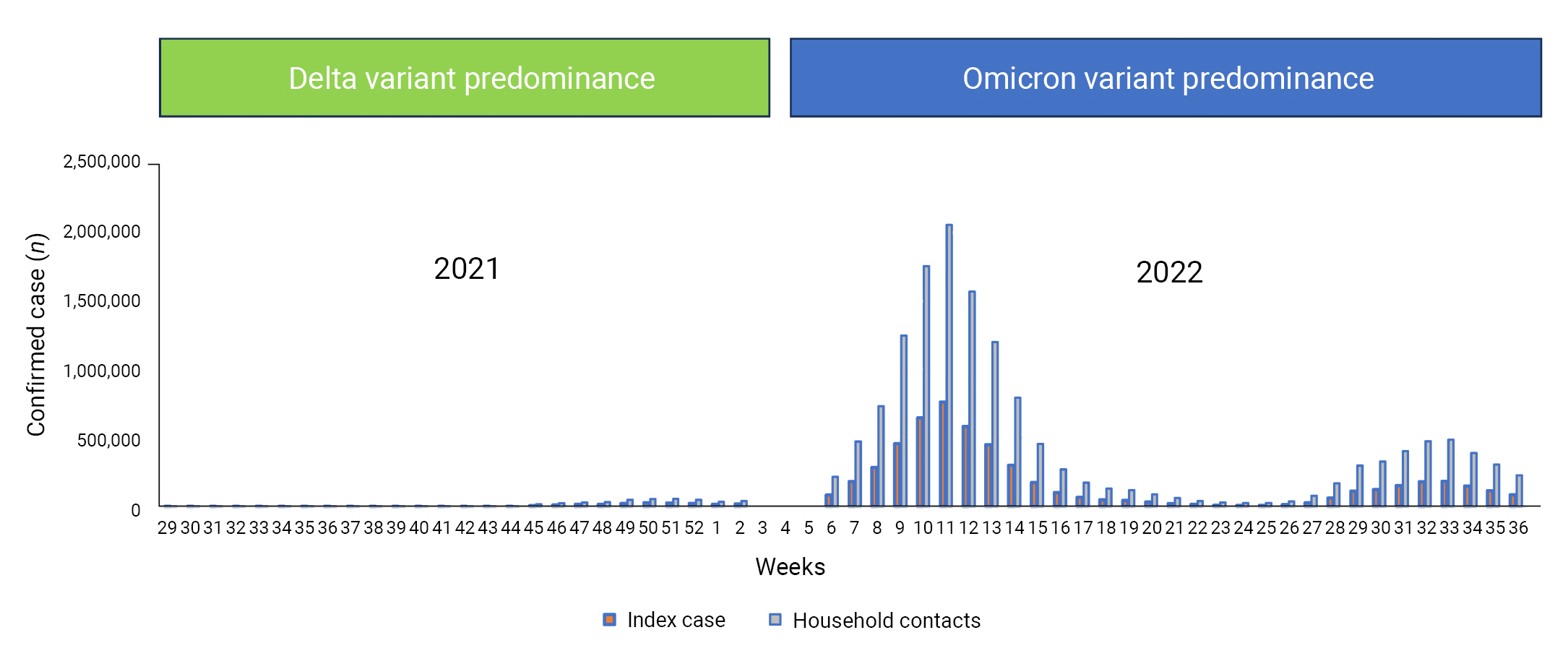
- Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has been prevalent worldwide since January 2020, with more than 640 million confirmed cases and 6.64 million deaths as of December 28, 2022. In the Republic of Korea (hereinafter, Korea), as of December 28, 2022 the number of confirmed COVID-19 cases was 28.6 million, with 31,774 deaths [1]. The COVID-19 epidemic in Korea is classified into 4 periods according to the circulation of SARS-CoV-2 variants of concern (VOCs), based on the detection of a relative share of more than 50% by whole-genome sequencing: pre-Delta variant predominance (January 20, 2020 to July 24, 2021), Delta variant predominance (July 25, 2021 to January 15, 2022), Omicron BA.1/BA.2 subvariant predominance (January 16 to July 23, 2022), and Omicron BA.5 subvariant predominance (July 24 to September 3, 2022) (Figure 1) [2]. Before the period of Delta variant predominance, other VOCs such as the Beta and Gamma variants were introduced into Korea, but the transmissibility of these variants was relatively low, with detection rates of less than 50% [3].
- After the introduction of new VOCs such as Delta and Omicron into Korea, the characteristics of community transmission of COVID-19 changed. Because those 2 variants were more transmissible than previous VOCs, several sporadic outbreaks were reported in the community with rapid surges of newly confirmed COVID-19 cases [4,5]; the same trend was reported worldwide [6]. Thus, the rate of transmissibility was shown to differ depending on the emergence of new SARS-CoV-2 VOCs. The secondary attack rate (SAR) is an important indicator for estimating community transmission. In the United Kingdom (UK), the household SAR of Delta variant cases was 1.7 times higher than that of Alpha variant cases [7]. In Norway, the household contacts of Omicron variant cases showed a SAR 1.3 times higher than that of Delta variant cases [8]. A Korean study also demonstrated a SAR 1.08 times higher for the Omicron variant than for the Delta variant [9]. In this regard, the household SAR for specific SARS-CoV-2 variants can be a significant indicator for measuring characteristics of community transmission of COVID-19 [10]. However, a study on household SARs across different periods of variant predominance has been difficult to undertake with a large dataset until recently. The current study aimed to characterize transmission patterns by comparing the household SARs and identifying risk factors during the periods of Delta and Omicron variant predominance in Korea.
Introduction
- Study Population
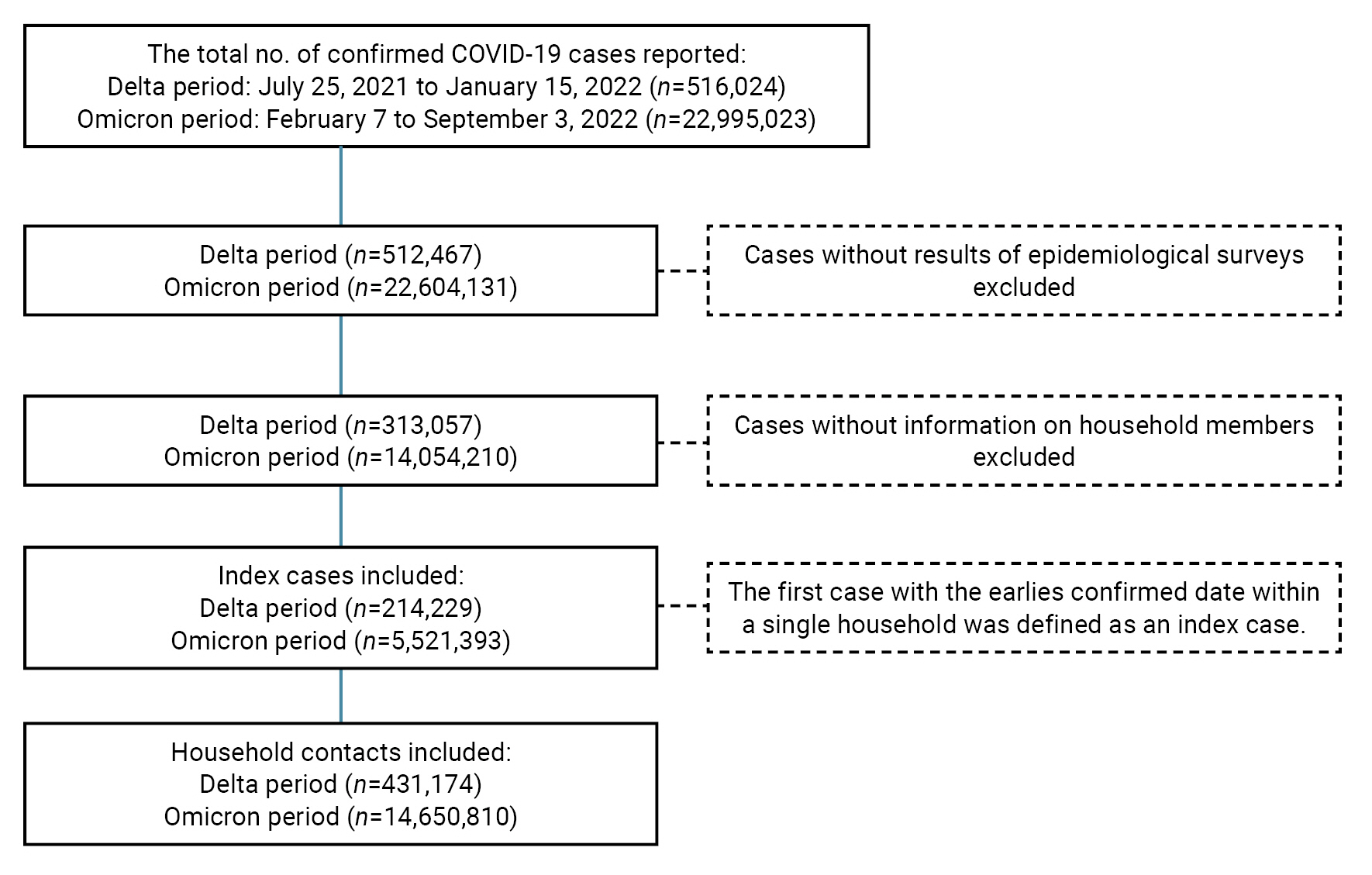
- Data were analyzed from confirmed COVID-19 cases and household contacts that were reported to the COVID-19 Information Management System (CIMS, https://covid19.kdca.go.kr), managed by Korea Disease Control and Prevention Agency (KDCA) as per the Infectious Disease Control and Prevention Act. We defined the period of Delta variant predominance (Delta period) from July 25, 2021 to January 15, 2022, and the period of Omicron variant predominance (Omicron period) from January 16 to September 3, 2022 [2]; for the Omicron period, data reporting began on February 7, 2022, when the KDCA introduced a mobile-based self-reporting form for epidemiological investigation [11]. During the Delta period, contacts of confirmed COVID-19 cases were enrolled in the CIMS and required to undergo polymerase chain reaction tests with 14 days of home quarantine [12]. During the Omicron period, contacts of confirmed cases were advised to monitor their health conditions, and public health centers provided all household contacts with education regarding home quarantine guidelines [13].
- The CIMS confirmed a total number of 516,024 cases for the Delta period and 22,995,023 cases for the Omicron period. Of these, cases without information on epidemiological surveys and household members were excluded from our analysis: 313,057 and 14,054,210 cases for the Delta and Omicron periods, respectively. The first case with the earliest confirmed date of COVID-19 infection within a single household was defined as an index case. The numbers of index cases and household contacts selected in this study were 214,229 and 431,174, respectively, for the Delta period, and 5,521,393 and 14,650,810, respectively, for the Omicron period (Figure 2).
- Data Sources
- Data on confirmed COVID-19 cases were retrieved from the CIMS. Information on household contacts was collected from the Epidemiological Investigation System. COVID-19 vaccination information was retrieved from the COVID-19 Vaccination Management System. The COVID-19 infection status such as second infection was assessed based on the definition used in the National Guidelines for Response to COVID-19 [12,13].
- Statistical Analysis
- Descriptive statistical analysis was performed to estimate the SARs during the Delta and Omicron periods, and the SARs were compared by sex, age, vaccination status, and infection status. Logistic regression was conducted to identify risk factors and to determine their adjusted odds ratios (aORs) and 95% confidence intervals (CIs). All analyses were conducted using SAS ver. 9.4 (SAS Institute).
- Ethical Considerations
- Information about all study participants was obtained after obtaining consent, in accordance with the Infectious Diseases Control and Prevention Act. The study was exempted from review by the Public Institutional Review Board designated by the Ministry of Health and Welfare (No. P01-202305-01-020).
Materials and Methods
- The total numbers of index cases and household contacts during the Delta period were 214,229 and 431,174, respectively. Of these, 151,627 cases were confirmed, with a SAR of 35.2%. Among both index cases and household contacts, the proportion was higher in males than in females. By age group, the largest proportions were those aged 0 to 18 years (23.5%) among index cases, 19 to 49 years (37.7%) among household contacts, and 19 to 49 years (35.3%) among confirmed cases. Slightly over half of the study population had received a second vaccine dose, and the vast majority had experienced only an initial infection (Table 1).
- During the Omicron period, the total numbers of index cases and household contacts were 5,521,393 and 14,650,810, respectively, of which 6,313,476 were confirmed cases. The SAR was 43.1%. Among both index cases and household contacts, the proportion of females was higher than that of males. By age group, the largest proportions were those aged 19 to 49 years (48.7%) among index cases, 19 to 49 years (47.8%) among household contacts, and 0 to 18 years (43.8%) among confirmed cases. A plurality of the study population had received a third vaccine dose, and the vast majority had experienced only an initial infection (Table 2).
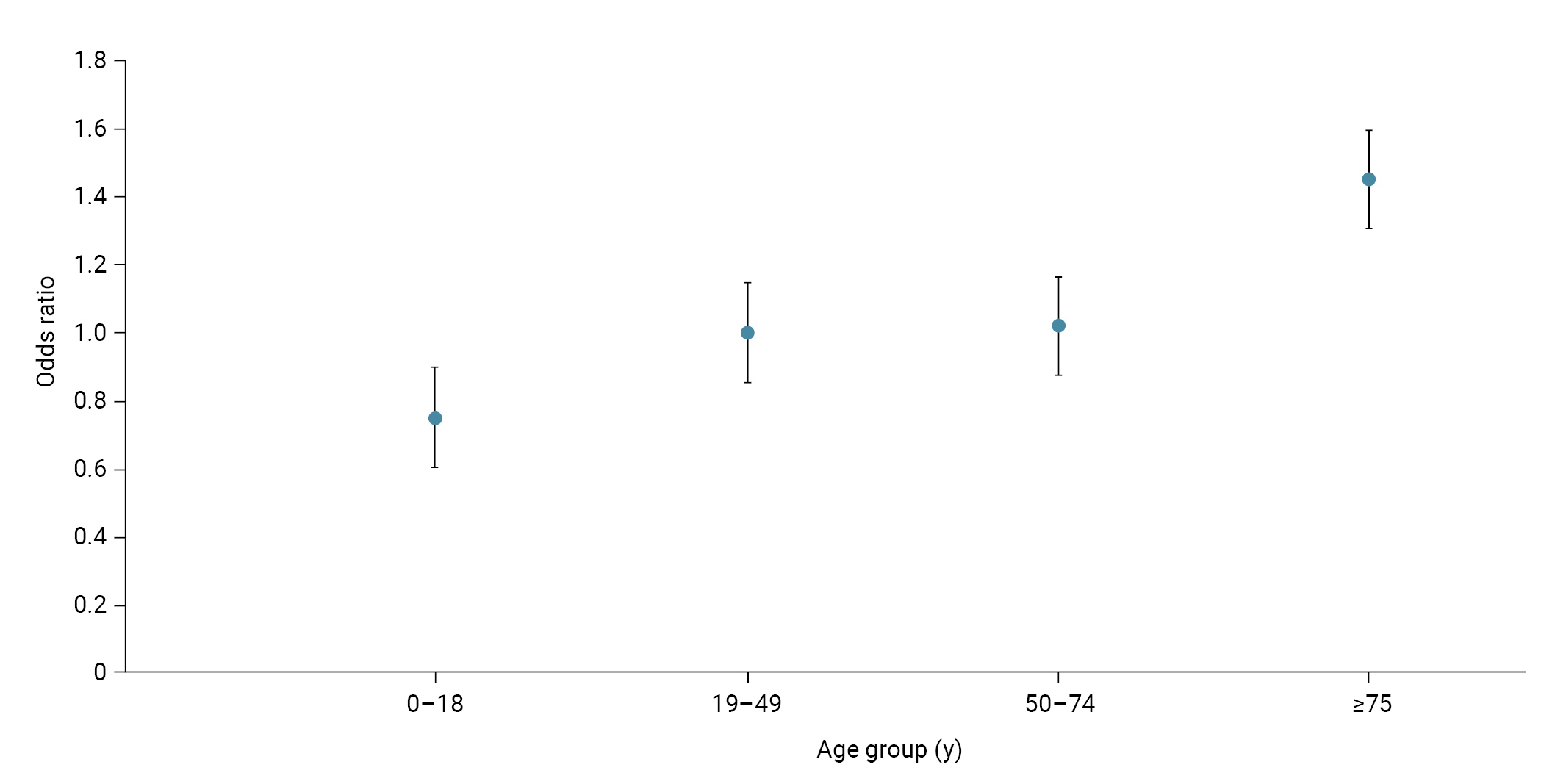
- The SAR among individuals aged ≥75 years (41.2%) was the highest during the Delta period, followed by individuals aged 0 to 18 years (37.8%). Unvaccinated individuals had the highest SAR (38.2%), and those experiencing an initial infection (35.2%) showed a higher SAR than those with a second infection (34.9%). The aOR of infection was 1.50 (95% CI, 1.46–1.54) in those aged 0 to 18 years and 1.17 (95% CI, 1.12–1.21) in those aged ≥75 years, compared to those aged 19 to 49 years. The aOR of infection according to vaccination status was 0.85 (95% CI, 0.81–0.88) after dose 1, 0.88 (95% CI, 0.86–0.90) after dose 2, and 0.65 (95% CI, 0.53–0.80) after dose 3, compared to unvaccinated individuals. The aOR of infection for individuals with a second infection was 0.96 (95% CI, 0.90–1.01) compared to those with an initial infection (Table 3; Figure 3).
- During the Omicron period, the SAR in males (44.4%) was higher than that in females (41.8%). By age group, those aged 0 to 18 years (50.1%) had the highest SAR, followed by the group aged ≥75 years (47.0%). The SARs for unvaccinated individuals and those experiencing an initial infection were the highest, accounting for 55.7% and 43.7%, respectively. The aOR of infection among those aged ≥75 years was 1.45 (95% CI, 1.44–1.47) compared to those aged 19 to 49 years. The aOR of infection by vaccination status was 0.83 (95% CI, 0.82–0.84) after dose 1, 0.72 (95% CI, 0.72–0.73) after dose 2, and 0.55 (95% CI, 0.55–0.55) after dose 3, compared to unvaccinated individuals. The aOR of infection for individuals with a second infection was 0.19 (95% CI, 0.19–0.20) and for a third infection was 0.16 (95% CI, 0.15–0.18), compared to an initial infection (Table 4; Figure 4).
Results
- Our study estimated the household SARs of index cases during the periods of Delta and Omicron predominance in Korea in terms of specific risk factors. Other studies reported SARs of 35% to 36% for the Delta variant and 49% to 53% for the Omicron variant, respectively [14,15]; our study found a similar SAR for the Delta variant (35.2%) but a slightly lower SAR for the Omicron variant (43.1%). The SAR of the Omicron period was 1.2 times higher than that of the Delta period in our study, which is consistent with the previous finding that reported a SAR 1.4 times higher for the Omicron variant [8,16]. This increase in SAR can be attributed to the improvement in diagnostic procedures and tools, which enabled people to have easy access to the tests over time [17].
- Compared to individuals aged 19 to 49 years, the aOR of infection was 1.50 times higher in those aged 0 to 18 years and 1.17 times higher in those aged ≥75 years during the Delta period, and 1.45 times higher in those aged ≥75 years during the Omicron period. These results agreed with studies conducted in other countries; unvaccinated individuals aged ≤19 years had a risk of infection 1.3 times higher compared to individuals aged 20 to 59 years in Japan [18,19]. Furthermore, individuals in the UK aged ≥75 years had a risk of infection 1.61 times higher compared to those aged 30 to 39 years [16]. This may be because children aged 0 to 11 years and older adults aged ≥75 years are more likely to spend time with family compared to other age groups; similarly, a Japanese study reported that the higher risk of infection for individuals aged ≤19 years owes to various factors such as spending longer periods of time with household members [18,19]. In Korea, COVID-19 vaccination was not yet authorized for children and adolescents aged 0‒17 years during the Delta period [20–23]. Furthermore, older individuals lose vaccine-induced immunity more rapidly than younger individuals, although they are likely to have better vaccine coverage [24,25].
- This study confirmed, similar to previous findings, that the SAR of unvaccinated groups was higher than that of vaccinated groups during both the Delta and Omicron periods [14,16]. Other countries reported a risk of infection 1.4 to 2.5 times higher among unvaccinated groups [8,16,18,19], which agrees with a study that showed a higher risk of infection among unvaccinated Koreans [9]. Additionally, a study conducted in Spain reported that COVID-19 vaccination was effective during the Delta period [26]. However, another Spanish cohort study confirmed that a third dose of mRNA-based COVID-19 vaccination was moderately effective in preventing infection by the Omicron variant for one month, with lower efficacy compared to the Delta period [27].
- During both Delta and Omicron periods, the SARs for individuals experiencing their second and third infections were higher, with higher risks of infection, compared to those with initial COVID-19 infection. Animal experimental studies have demonstrated that reinfected animals did not shed the virus at sufficient levels to infect naïve animals, with no nasal and oral shedding of the virus [28,29], and evidence on transmissibility among reinfected patients after their initial infection with COVID-19 has been inconclusive. Therefore, additional investigation of transmission among reinfected COVID-19 patients may be required [30].
- Although a previous study investigated SARs in Korea by COVID-19 vaccination status, the result predated the Omicron period and did not analyze risk factors such as reinfection and age group [9]. The current study used the large datasets reported to the CIMS during the Delta and Omicron periods, identifying risk factors such as age group, vaccination status, and reinfection. Children and adolescents, who are more susceptible to becoming infected with SARS-CoV-2, may have lower transmissibility without being vaccinated [25,31,32], and older individuals may be more likely to develop symptoms, since they have lower vaccine-induced immunity compared to young adults [25,32]. Furthermore, vaccinated individuals may be less likely to practice social distancing than unvaccinated people [33]. In this respect, other aspects should be considered in interpreting the effects of risk factors. Since the World Health Organization has recently lifted the Public Health Emergency of International Concern for COVID-19, a follow-up study on risk factors such as reinfection needs to be conducted to provide evidence-based guidelines [34]. We hope that the results of this study can help develop age-specific immunization plans and responses to reduce the SAR in preparing for emerging infectious diseases or potential new VOCs of SARS-CoV-2.
- This study has some limitations. First, because COVID-19 rapid antigen tests became available to use for confirmation during the Omicron period, limiting to the identification of variant types, we were not able to include all confirmed COVID-19 cases in Korea during the Delta and Omicron periods. Therefore, the result might not be representative of the entire population. Second, our analysis included only the number of vaccine doses administered without accounting for their type or the time elapsed following vaccination. Third, isolation for close contacts was not required during the Omicron period; thus, those contacts might have had a risk of infection through community transmission.
Discussion
- • This study analyzed the household secondary attack rates (SAR) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and identified risk factors during the periods of Delta and Omicron variant predominance in the Republic of Korea.
- • The SAR was 35.2% for the Delta period and 43.1% for the Omicron period. Persons aged 0 to 18 years and ≥75 years, unvaccinated individuals, and individuals experiencing initial infection had an increased risk of infection with SARS-CoV-2.
HIGHLIGHTS
-
Ethics Approval
Information about all study participants was obtained after obtaining consent in accordance with the Infectious Diseases Control and Prevention Act. The study was exempted from review by the Public Institutional Review Board designated by the Ministry of Health and Welfare (No. P01-202305-01-020).
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The data used in this study are protected under the Personal Information Protection Act.
-
Authors’ Contributions
Conceptualization: JL, MK, DL; Data curation: JL, SK, DL; Formal analysis: JL, GP; Methodology: SEL, JL, DL; Project administration: JL; Writing–original draft: JL; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Article information
-
Acknowledgements
- The authors appreciate the Division of Emerging Infectious Disease Response, KDCA for making this study possible.


- 1. Korea Disease Control and Prevention Agency (KDCA). Current COVID-19 outbreak situation [Internet]. KDCA; 2022 [cited 2023 Apr 12]. Available from: https://ncov.kdca.go.kr/tcmBoardView.do?brdId=3&brdGubun=30&dataGubun=&ncvContSeq=7050&concont=7050&board_id=301&gubun=ALL#. Korean.
- 2. Ryu B, Shin E, Kim NY, et al. Severity of COVID-19 associated with SARS-CoV-2 variants circulating in the Republic of Korea. Public Health Wkly Rep 2022;15:2873−95. Korean, English.Article
- 3. Kim IH, Park AK, Lee H, et al. Status and characteristics of the SARS-CoV-2 variant outbreak in the Republic of Korea in January 2021. Public Health Wkly Rep 2022;15:497−510. Korean.
- 4. Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis 2022;22:35−42.PubMedPMC
- 5. GISAID. Tracking of hCoV-19 variants [Internet]. GISAID; 2022 [updated 2022 Oct 6; cited 2022 Oct 6]. Available from: https://gisaid.org/hcov19-variants/.
- 6. Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 Omicron variant severity in Ontario, Canada. JAMA 2022;327:1286−8.ArticlePubMedPMC
- 7. Allen H, Vusirikala A, Flannagan J, et al. Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): national case-control study. Lancet Reg Health Eur 2022;12:100252. ArticlePubMedPMC
- 8. Jalali N, Brustad HK, Frigessi A, et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat Commun 2022;13:5706. ArticlePubMedPMCPDF
- 9. Park H, Park YJ, Lee SE, et al. mRNA vaccine effectiveness against SARS-CoV-2 B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant transmission from home care cases to household contacts in South Korea. Osong Public Health Res Perspect 2022;13:435−42.ArticlePubMedPMCPDF
- 10. Haroon S, Chandan JS, Middleton J, et al. Covid-19: breaking the chain of household transmission. BMJ 2020;370:m3181. ArticlePubMed
- 11. Korea Disease Control and Prevention Agency (KDCA). Press releases: COVID-19 outbreak and vaccination status (2.8) [Internet]. KDCA; 2022 [cited 2022 Mar 20]. Available from: https://ncov.kdca.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6364&contSeq=6364&board_id=312&gubun=ALL. Korean.
- 12. Korea Disease Control and Prevention Agency (KDCA). Guidelines for response to coronavirus disease-19 (for local government). 10-1st ed. KDCA; 2021. Korean.
- 13. Korea Disease Control and Prevention Agency (KDCA). Guidelines for response to coronavirus disease-19 (for local government). 12th ed. KDCA; 2022. Korean.
- 14. Watanapokasin N, Siripongboonsitti T, Ungtrakul T, et al. Transmissibility of SARS-CoV-2 variants as a secondary attack in Thai households: a retrospective study. IJID Reg 2021;1:1−2.ArticlePubMedPMC
- 15. Baker JM, Nakayama JY, O'Hegarty M, et al. SARS-CoV-2 B.1.1.529 (Omicron) variant transmission within households - four U.S. jurisdictions, November 2021-February 2022. MMWR Morb Mortal Wkly Rep 2022;71:341−6.ArticlePubMedPMC
- 16. Allen H, Tessier E, Turner C, et al. Comparative transmission of SARS-CoV-2 Omicron (B. 1.1. 529) and Delta (B. 1.617. 2) variants and the impact of vaccination: national cohort study, England. Epidemiol Infect 2023;151:e58.ArticlePubMedPMC
- 17. Madewell ZJ, Yang Y, Longini IM Jr, et al. Factors associated with household transmission of SARS-CoV-2: an updated systematic review and meta-analysis. JAMA Netw Open 2021;4:e2122240.ArticlePubMedPMC
- 18. Ogata T, Tanaka H, Nozawa Y, et al. Increased secondary attack rate among unvaccinated household contacts of coronavirus disease 2019 patients with Delta variant in Japan. Int J Environ Res Public Health 2022;19:3889. ArticlePubMedPMC
- 19. Ogata T, Tanaka H, Tanaka E, et al. Increased secondary attack rates among the household contacts of patients with the Omicron variant of the coronavirus disease 2019 in Japan. Int J Environ Res Public Health 2022;19:8068. ArticlePubMedPMC
- 20. Korea Disease Control and Prevention Agency (KDCA). Press releases: adolescents aged 16-17 years vaccination (October 18, regular briefing) [Internet]. KDCA; 2021 [cited 2022 May 12]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=717277&cg_code=C01&act=view&nPage=1. Korean.
- 21. Korea Disease Control and Prevention Agency (KDCA). Press releases: adolescents aged 12-17 years vaccination (August 30, regular briefing) [Internet]. KDCA; 2021 [cited 2022 May 12]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=716707&cg_code=C0&act=view&nPage=11. Korean.
- 22. Korea Disease Control and Prevention Agency (KDCA). Press releases: children aged 5-11 years vaccination (March 24, regular briefing) [Internet]. KDCA; 2021 [cited 2022 May 12]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=719074&cg_code=&act=view&nPage=1. Korean.
- 23. Korea Disease Control and Prevention Agency (KDCA). Press releases: toddler aged 0-4 years vaccination (January 27, Friday) [Internet]. KDCA; 2023 [cited 2023 May 6]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=721767&cg_code=&act=view&nPage=18. Korean.
- 24. Brockman MA, Mwimanzi F, Lapointe HR, et al. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. J Infect Dis 2022;225:1129−40.ArticlePubMedPMCPDF
- 25. Bajaj V, Gadi N, Spihlman AP, et al. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol 2021;11:571416. ArticlePubMedPMC
- 26. Lopez-Munoz I, Torrella A, Perez-Quilez O, et al. SARS-CoV-2 secondary attack rates in vaccinated and unvaccinated household contacts during replacement of Delta with Omicron variant, Spain. Emerg Infect Dis 2022;28:1999−2008.ArticlePubMedPMC
- 27. Monge S, Rojas-Benedicto A, Olmedo C, et al. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis 2022;22:1313−20.PubMedPMC
- 28. Gaudreault NN, Carossino M, Morozov I, et al. Experimental re-infected cats do not transmit SARS-CoV-2. Emerg Microbes Infect 2021;10:638−50.ArticlePubMedPMC
- 29. Brustolin M, Rodon J, Rodriguez de la Concepcion ML, et al. Protection against reinfection with D614- or G614-SARS-CoV-2 isolates in golden Syrian hamster. Emerg Microbes Infect 2021;10:797−809.ArticlePubMedPMC
- 30. Ren X, Zhou J, Guo J, et al. Reinfection in patients with COVID-19: a systematic review. Glob Health Res Policy 2022;7:12. ArticlePubMedPMCPDF
- 31. Heavey L, Casey G, Kelly C, et al. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Euro Surveill 2020;25:2000903. ArticlePubMedPMC
- 32. Andersson O, Campos-Mercade P, Meier AN, et al. Anticipation of COVID-19 vaccines reduces willingness to socially distance. J Health Econ 2021;80:102530. ArticlePubMedPMC
- 33. Cortis D. On determining the age distribution of COVID-19 pandemic. Front Public Health 2020;8:202. ArticlePubMedPMC
- 34. Korea Disease Control and Prevention Agency (KDCA). Press releases: World Health Organization (WHO) announces the lifting of the COVID-19 ‘International Public Health Emergency’ (May 6, Saturday) [Internet]. KDCA; 2023 [cited 2023 May 12). Available from: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=722478&cg_code=&act=view&nPage=1. Korean.
References
Figure & Data
References
Citations



 Cite
Cite