Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 13(3); 2022 > Article
-
Review Article
Effect of clofibrate on reducing neonatal jaundice: a systematic review and meta-analysis -
Fatemeh Eghbalian1
 , Lotfollah Karimi2
, Lotfollah Karimi2 , Roya Raeisi1
, Roya Raeisi1 , Ayda Hasanpour Dehkordi3
, Ayda Hasanpour Dehkordi3 , Hamid Bouraghi4
, Hamid Bouraghi4
-
Osong Public Health and Research Perspectives 2022;13(3):174-183.
DOI: https://doi.org/10.24171/j.phrp.2021.0336
Published online: June 30, 2022
1Department of Pediatrics, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Basic Science, Hamedan University of Technology, Hamedan, Iran
3Departments of Psychiatric, Faculty of Medical Sciences, Islamic Azad University, Khomein, Iran
4Department of Health Information Technology, School of Paramedical Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
- Corresponding author: Roya Raeisi Department of Pediatrics, Hamadan Beasat Hospital, Hamadan University of Medical Sciences, Hamadan 6516845436, Iran E-mail: r_reisi2@yahoo.com
© 2022 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 2,751 Views
- 105 Download
Abstract
- In neonates, bilirubin tends to be deposited in body tissues, especially the skin and mucous membranes. Jaundice is an early symptom of bilirubin excretion disorders. Therefore, the aim of this study was to investigate the effect of clofibrate on reducing neonatal jaundice. In this systematic review, international databases, including PubMed, Scopus, Web of Science, Embase, Cochrane, and Google Scholar, were searched without time and language restrictions. The reference lists of all studies ultimately included were manually searched. In the 17 articles reviewed, with a sample size of 665 people published between 2005 and 2019, the average weight of the neonates varied from 2,186 g to 4,000 g. Furthermore, the average age of neonates varied from 2 days to 9 days. Four doses of clofibrate (25, 30, 50, 100 mg/kg of neonatal body weight) were used. The bilirubin level of neonates significantly decreased in the intervention group 24, 36, 48, and 72 hours after the start of treatment. Clofibrate administration decreased total serum bilirubin, especially from the second day onwards, and also reduced hospitalization time, hospital costs, and side effects from hospitalization.
- Bilirubin is a compound produced by the catabolism of hemoglobin that tends to be deposited in the body tissues of neonates, especially the skin and mucous membranes. Jaundice is an early symptom of bilirubin excretion disorders. If bilirubin is not excreted from the body in time, it will reside in the brain tissue and cause temporary and permanent neurological disorders by damaging the brainstem, in a condition known as kernicterus [1]. Elevated bilirubin levels (hyperbilirubinemia) are among the most common disorders in infancy, observed in 60% of infants, of whom 10% have the severe form for which treatment is needed [2]. The rate of hyperbilirubinemia in premature neonates is 80% [3], and neonatal jaundice is observed in up to 85% of all live births. If there is no hemolysis, sepsis, trauma at birth, or prematurity, it usually resolves within 3 to 5 days without significant complications. However, evidence suggests that severe neonatal jaundice (SNJ) leads to substantial morbidity and mortality. SNJ is also an important cause of neurological disorders and other long-term consequences, including cerebral palsy, non-syndromic auditory neuropathy, deafness, and learning difficulties. A recent report by Slusher et al. [4] noted that at least 481,000 term/near-term neonates are affected by SNJ/hyperbilirubinemia each year, of whom 114,000 die and more than 63,000 survive with kernicterus. In the USA, jaundice is seen in 15.6% of hospitalized neonates. The incidence of SNJ is 667.8 per 10,000 live births in Africa and 3.7 per 10,000 live births in Europe [5]. Although this disorder generally resolves, an appropriate intervention to avoid bilirubin neurotoxicity should be seriously considered [6]. There are 3 ways to treat hyperbilirubinemia: phototherapy, blood transfusion, and medication. The most common of these methods is phototherapy, and transfusion of the baby's blood in conditions of excessive bilirubin [7–10]. Although blood transfusion is one of the main treatments for hyperbilirubinemia, it has some side effects [11,12]. These detrimental side effects suggest that an alternative drug treatment strategy should be developed to address this disorder. Drug interventions include D-penicillamine, phenobarbital, clofibrate, bile salts, metalloporphyrins, laxatives, and bilirubin oxidases [6,13–15]. Clofibrate is a glucuronosyltransferase inducer suggested to reduce bilirubin levels in neonates with hyperbilirubinemia [16–21]. Clofibrate is an activator of peroxisome proliferator-activated receptors (PPARs) that effectively lowers cholesterol and triglyceride levels in adults. It also induces glucuronyl transferase and causes the accumulation and excretion of bilirubin. Some studies have also suggested reducing neonates' need for phototherapy and shortening their hospitalization course [11,17–21]. Clofibrate is a practical drug recommended for the treatment of neonatal hyperbilirubinemia. Research results have shown that clofibrate effectively reduces neonatal jaundice, with an effect appearing 24 hours after treatment [16,19–21]. A single dose of 50 mg/kg of clofibrate to treat neonatal hyperbilirubinemia shortens the hospitalization course, which is also economically advantageous [6]. Clofibrate may have short-term benefits for neonates with hyperbilirubinemia, especially for term neonates and neonates without hemolytic diseases [19–22]. Clofibrate results in a faster reduction of total serum bilirubin (TSB) and a shorter hospitalization course, and no side effects have been observed in full-term neonates with jaundice [7,16,19–21].
- A recent Cochrane review on clofibrate administration as an adjunct to phototherapy for neonatal unconjugated hyperbilirubinemia was limited due to a high degree of heterogeneity among the trials, a lack of trials from different geographical regions, and a lack of data on mortality from kernicterus and long-term safety [23]. Therefore, the present study aimed to evaluate the effect of clofibrate on the reduction of TSB and neonatal jaundice.
Introduction
- Study Protocol
- This systematic review and meta-analysis investigated the effect of clofibrate on reducing neonatal jaundice. This study was written based on the PRISMA protocol for systematic review and meta-analysis studies.
- Statistical Population
- Studies in which neonates were treated with clofibrate to reduce their blood bilirubin levels in addition to phototherapy were evaluated. In selecting these people, no restrictions were imposed on sex, age, race, and weight at birth.
- Study Outcome
- The main outcome considered was a reduction in bilirubin levels.
- Search Strategy
- In this systematic review, the international databases, including PubMed, Scopus, Web of Science, Embase, Cochrane, and Google Scholar, were searched without time and language restrictions. If an article was published in a language other than English, the full text of the article was translated into Persian to extract the relevant information. The search was performed using the standard keywords "jaundice,” “icterus,” “hyperbilirubinemia,” “neonatal,” “infant,” “clofibrate,” “meta-analysis,” and “systematic review,” as well as their equivalent MeSH terms (updated through May 15, 2021). Their compounds were also searched in the abovementioned databases using the (AND, OR) operators. The reference lists of all studies that were ultimately included were also manually searched. Table 1 shows the search strategy in some databases and Table 2 presents the results for the evaluation of quality of studies [1,2,6–8,11,12,16–18,23–29].
- Inclusion and Exclusion Criteria
- The study population was all neonates who received clofibrate to reduce bilirubin levels in addition to phototherapy. The intervention was clofibrate administration. The comparison was neonates who used placebo in addition to phototherapy or received no treatment other than phototherapy. The study outcome was bilirubin.
- In this meta-analysis, the preliminary studies included randomized clinical trials with or without blinding. The intervention groups in the included trials were neonates who had received clofibrate in addition to phototherapy, and the control groups received no intervention or placebo.
- Exclusion criteria included failure to report the required information, case reports, low-quality studies based on the Cochrane Institute's clinical quality assessment checklist, lack of access to the full texts of studies, studies examining the effect of clofibrate on neonatal jaundice based on measurements of bilirubin levels in the umbilical cord of neonates, and those investigating the effect of clofibrate combined with another drug on neonatal jaundice.
- After identifying the initial studies, 2 authors independently evaluated all the initial studies using the Cochrane Institute's clinical quality assessment checklist. This checklist includes 7 items, each evaluating 1 of the dimensions or types of essential biases in clinical trials. Each item in this checklist has 3 options: a high risk of bias, a low risk of bias, and unclear. After completing the bias risk assessment in all studies, disagreements about the item options in each study were evaluated and resolved through consensus. Table 2 shows the quality evaluation of studies.
- Data Extraction
- Two researchers independently extracted data from studies to minimize reporting bias and data collection errors. The researchers entered the extracted data onto a checklist, including the first author’s name, the year of study publication, study title, the number of samples, the mean and standard deviation of neonatal bilirubin levels before and after the intervention, age, sex, and neonatal weight, clofibrate dosage, and follow-up duration. A third researcher reviewed the data extracted by the 2 previous researchers to correct any discrepancies. If the required data were not reported in an article, a request for the data was sent through correspondence with the article's author.
- Statistical Analysis
- Due to the quantitative nature of the outcome of interest, the effect size of the intervention was calculated based on the mean difference in serum bilirubin levels before and after the intervention compared to the mean difference outside the experimental group.
- Data from studies were combined for the meta-analysis according to the number of samples, mean, and standard deviation. In order to evaluate the heterogeneity of the studies, the Cochrane Q test and the I2 index were used. Since a fixed-effects model is used when heterogeneity is low and a stochastic-effects model is used when a high degree of heterogeneity is present, the present study used a stochastic-effects model. Data analysis was performed using Stata ver. 14.0 (StataCorp., College Station, TX, USA). A p-value <0.05 was considered to indicate statistical significance.
Materials and Methods
PICO (patient, intervention, comparison, outcome) components
Inclusion criteria for preliminary studies
Exclusion criteria
Quality evaluation of studies
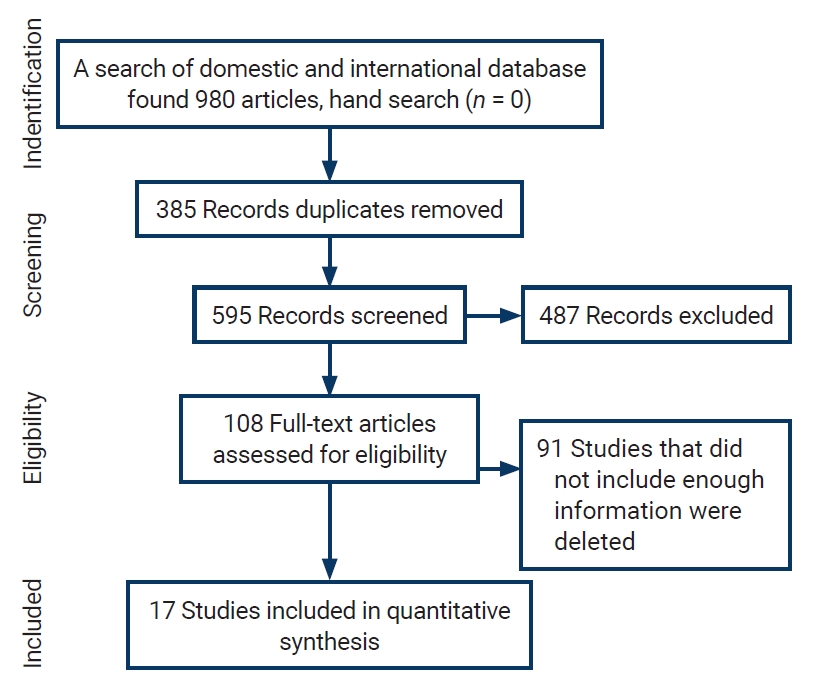
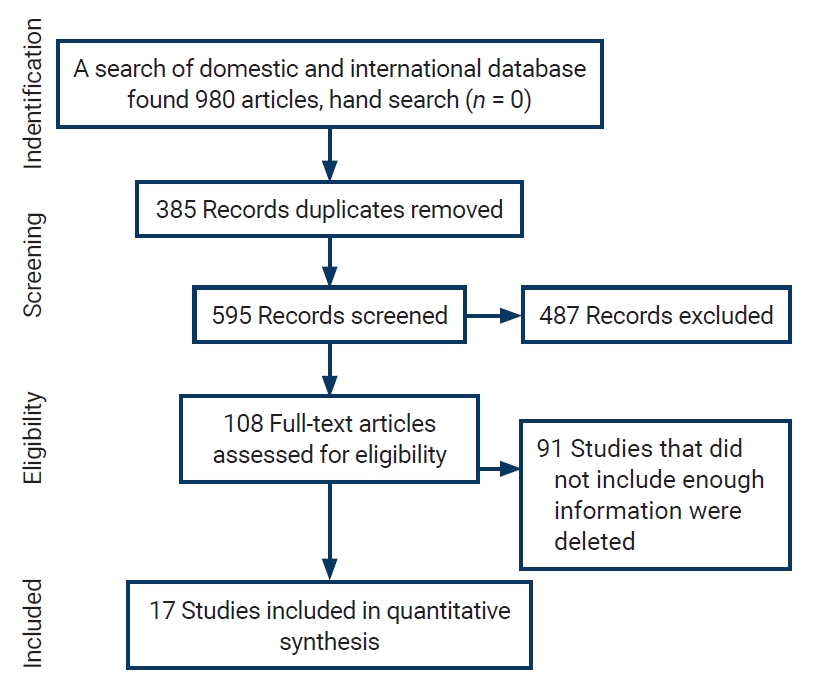
- Initially, 980 articles were found in the above databases. After reviewing the titles, 385 duplicate studies were excluded. The remaining 595 abstracts were reviewed, and 487 articles were eliminated according to the exclusion criteria. Ninety-one of the remaining 108 articles were removed due to incomplete information or the lack of a full text. Finally, the remaining 17 articles entered the quality evaluation stage; all of these articles were deemed to be of good quality and included in the meta-analysis. Figure 1 presents a flow chart of the inclusion of studies in the systematic review and meta-analysis.
- Characteristics of Studies Included in the Systematic Review
- Table 3 presents information on the articles entered into the systematic review and meta-analysis. In the 17 articles reviewed, with a sample size of 665 people published between 2005 and 2019, the average weight of the neonates varied from 2,186 g to 4,000 g. The average age of the neonates ranged from 2.09 days to 9.8 days. Four doses of clofibrate (25, 30, 50, 100 mg/kg of neonatal body weight) were used in the studies. All studies were conducted in Iraq, Iran, and India. Table 3 shows the characteristics of studies included in this systematic review [1,2,6–8,11,12,16–18,23–29].
- Since the study phases were different (6, 12, 16, 24, 36, 48, and 72 hours after the intervention), we could not conducted a subgroup analysis based on clofibrate dose, age, weight, or the countries studied.
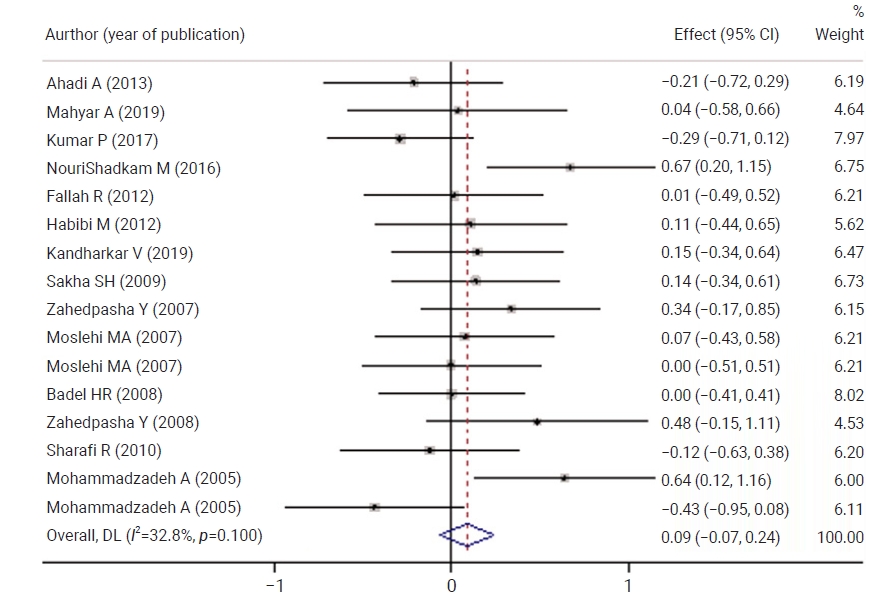
- Figure 2 shows a comparison of neonatal bilirubin levels between the control and case groups before the intervention There was no statistically significant difference between the control and case groups with respect to the mean scores of neonatal bilirubin levels. No statistically significant difference was observed between both groups in terms of neonatal bilirubin levels 6 hours after the intervention.
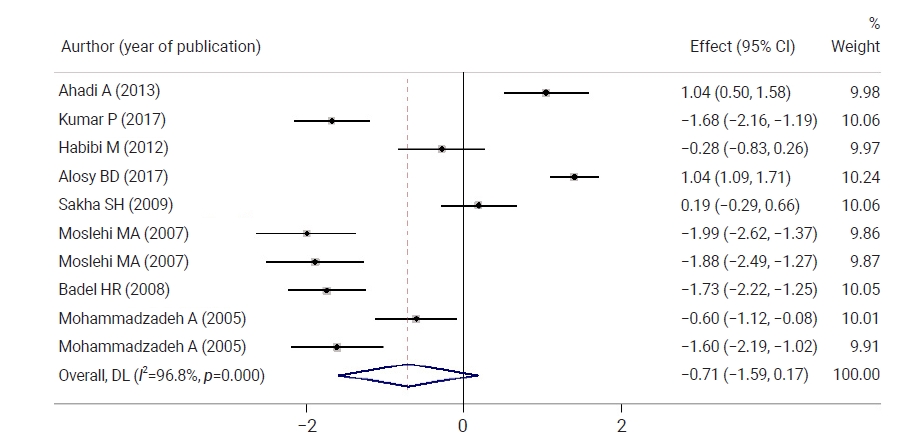
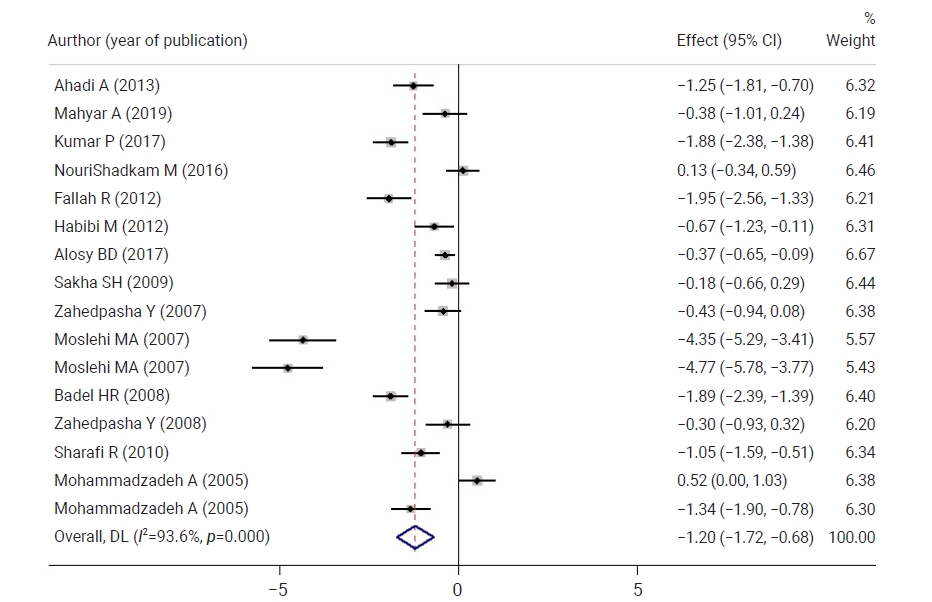
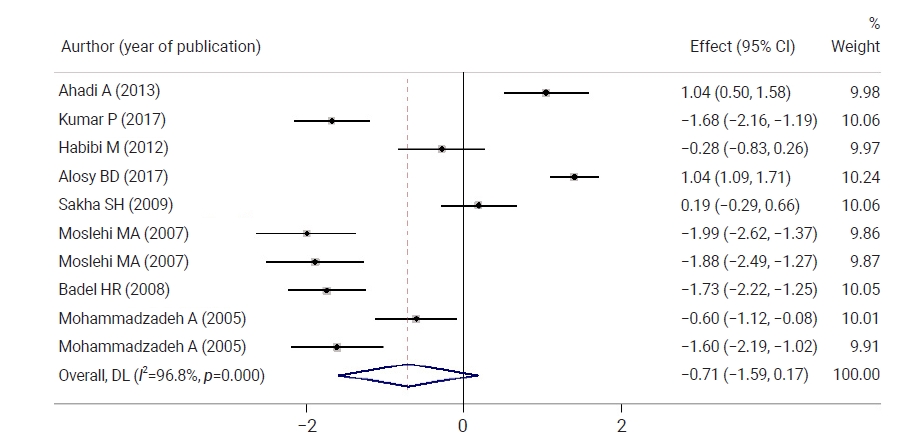
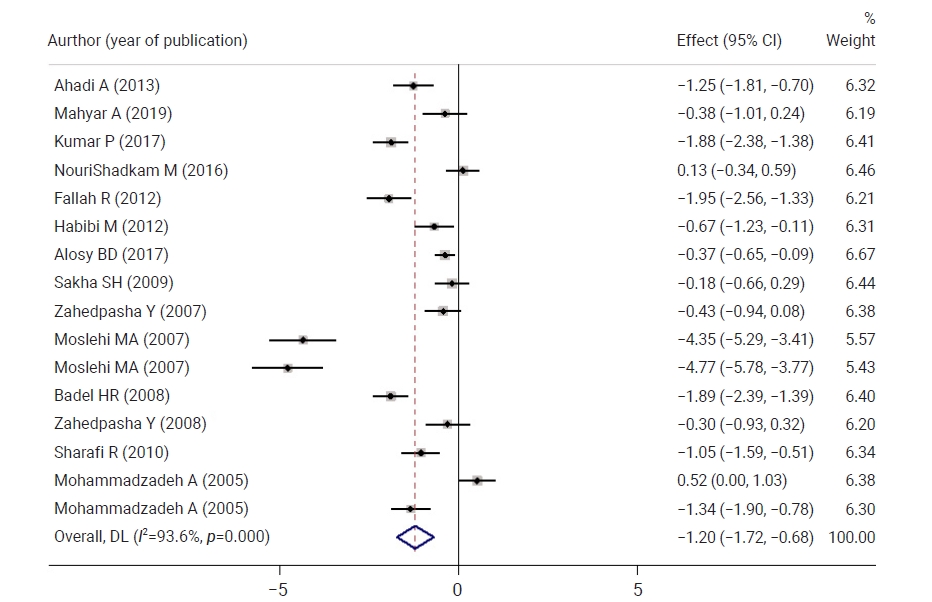
- Figure 3 shows a comparison of neonatal bilirubin levels between both groups 12 hours after the intervention. Neonatal bilirubin levels in the intervention group were 0.71 mg/dL lower than those in controls, but the difference was not statistically significant. Figure 4 shows a comparison of neonatal bilirubin levels between both groups 24 hours after the intervention. Clofibrate use significantly decreased the neonatal bilirubin levels in the intervention group by 1.20 mg/dL compared to the control group. A significant difference was observed between the control and intervention groups with respect to the mean neonatal bilirubin levels. The neonatal bilirubin levels in the intervention group were 2.39 mg/dL lower than those in the control group.
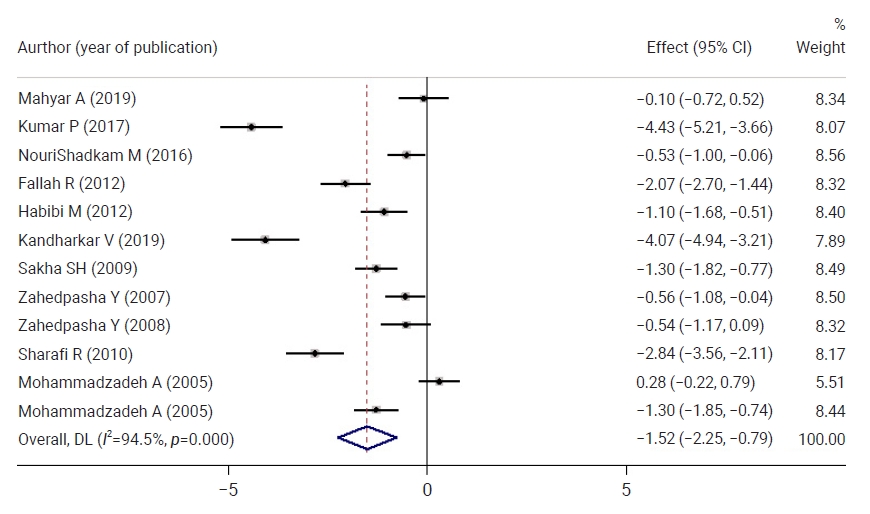
- Figure 5 shows a comparison of neonatal bilirubin levels between both groups 48 hours after the intervention. Neonatal bilirubin levels in the intervention group were 1.52 mg/dL lower than those of the control group, and this difference was statistically significant. At 72 hours after the intervention, neonatal bilirubin levels in the intervention group were 0.75 mg/dL lower than those in the control group, but this difference was not statistically significant.
- Neonatal bilirubin levels in the intervention group significantly decreased by 1.64 at 6 hours after the intervention, by 2.79 at 12 hours after the intervention, and by 1.81 at 16 hours after intervention. Two days after treatment, the neonatal bilirubin levels in the intervention group significantly decreased by 3.88 compared to the beginning of the study, and by 36 hours after the intervention, a significant decrease (by 4.01) in neonatal bilirubin levels was observed in the intervention group. By 48 hours, the neonatal bilirubin levels in the intervention group significantly decreased by 4.86 compared to before clofibrate consumption, and a 3.87 decrease was observed at 72 hours. In all phases, neonatal bilirubin levels decreased significantly in the intervention group compared to before the intervention
Results
- No statistically significant difference was observed between the control and intervention groups with respect to the mean neonatal TSB values before the intervention. Until 6 and 12 hours after the intervention, neonatal TSB levels decreased in both groups, without statistically significant between-group differences.
- Twelve hours after the intervention, neonatal TSB levels in the intervention group were 0.71 lower than those in the control group, which was not a statistically significant difference. The neonatal TSB levels in the intervention group were 0.54, 1.20, 2.39, 1.52, and 0.71 lower than those in the control group at 16, 18, 24, 36, 48, and 72 hours after the intervention, respectively, and these differences were statistically significant.
- The mean TSB level was significantly decreased by 1.64 at 12 hours after the intervention, 2.79 at 16 hours after the intervention, 1.81 at 24 hours after the intervention, 3.88 at 36 hours after the intervention, 4.01 at 48 hours after the intervention and 4.86 at 72 hours after the intervention in the intervention group. The neonatal TSB levels significantly decreased in the intervention group in all phases compared to before the intervention.
- The results reported by Caballero-Noguez et al. [30] regarding changes in total and indirect bilirubin levels in 2 groups (phenobarbital and clofibrate) compared to the control group showed that TSB levels significantly decreased 24 and 72 hours after the intervention. Alosy [24] showed that 12 hours, 24 hours, and 4 days after the intervention, TSB levels decreased in the clofibrate group and the control group, but this drop was more significant in the clofibrate group, which is consistent with our study. This may be due to the effect of clofibrate on enhancing glucuronyl transferase activity, as a result of which clofibrate raises hepatic bilirubin clearance in 6 hours. Unlike sodium phenobarbital, clofibrate does not cause sleepiness or respiratory depression. It also results in liver bilirubin clearance [16]. Fallah et al. [6] showed that 12 and 48 hours after the intervention, 27 neonates in the clofibrate group (90%) and 15 neonates in the control group (56.7%) had TSB levels less than 14 mg/dL (p=0.02). The mean length of hospitalization (mean±standard deviation: 1.7±0.7 days vs. 1.2±3.2 days; p=0.03) and the duration of phototherapy (mean±standard deviation: 30.2±13.99 hours vs. 46.2±58.5 hours; p=0.001) were significantly lower in the clofibrate group. Loose stool was only observed in 2 clofibrate patients. There was no significant difference in the safety of the treatments, and they showed that a dose of 50 mg/kg of clofibrate was effective in treating neonatal hyperbilirubinemia and reducing hospitalization duration and cost.
- TSB levels in the clofibrate group and phototherapy at 12, 24, 36, and 48 hours after the intervention were significantly lower than those in the phototherapy group. Significantly less phototherapy was needed in the clofibrate group than in the phototherapy-only group [1,19].
- Long-term administration of clofibrate in adults has several side effects, such as nausea and vomiting, loose stool, muscle cramps, and pruritus; however, no such effects have been reported in neonates who receive high doses of clofibrate [1,11,25].
- Studies have shown that both clofibrate and phenobarbital reduce TSB levels in a single dose, but phenobarbital is more effective; therefore, phenobarbital reduces TSB levels more rapidly than clofibrate, thereby reducing hospitalization time and costs [24].
- Studies have shown that in addition to reducing TSB levels, clofibrate also shortens the duration of phototherapy. Clofibrate may have short-term benefits in full-term infants who do not have a hemolytic disease; however, long-term follow-up is required to evaluate its safety and long-term effects. At present, there is no evidence suggesting that clofibrate can alter the likelihood of death and kernicterus [25].
- Habibi et al. [16] showed that clofibrate reduced TSB 24 hours after administration, while in other studies, it was effective 12 and 16 hours after clofibrate administration. Clofibrate activates PPARs and regulates plasma lipid levels by lowering very-low-density lipoproteins. The drug is absorbed from the gastrointestinal tract and rapidly hydrolyzed to an active metabolite (clofibric acid). This active metabolite is ultimately excreted through urine as conjugated glucuronide. Sakha et al. [26] also showed that clofibrate is an effective supplementary drug in neonatal hyperbilirubinemia, leading to a decrease in TSB levels and a reduction in the phototherapy duration in full-term and premature neonate. Kumar et al. [23] and Kandharkar [27]observed that clofibrate administration significantly reduced serum TSB levels at 48 hours after the intervention. Mahyar et al. [12] showed that purgative manna and clofibrate did not reduce TSB levels in unconjugated hyperbilirubinemia neonates, which is inconsistent with our study and previous studies.
- Eghbalian et al. [31] showed that the prescription of 25 mg/kg clofibrate as a single dose, just as the dose of 50 mg/kg as a single dose, could significantly reduce serum bilirubin levels. These researchers recommended using a low dose of clofibrate to treat neonatal unconjugated hyperbilirubinemia.
- Gholitabar et al. [32] argued that the existing data have been insufficient to absolutely confirm the effect of clofibrate on neonatal jaundice, and more studies are needed to be conducted on this issue. Clofibrate is an available drug that can effectively treat neonatal hyperbilirubinemia without any side effects. However, the general administration of this drug in high-risk neonates, such as premature infants and those with hemolytic jaundice should be further investigated [11].
- Moslehi and Pishva [17] showed no statistically significant difference between a low dose (25 mg/kg) and a moderate dose (50 mg/kg) of clofibrate. Six hours after clofibrate administration, indirect TSB levels decreased compared with the control group. Clofibrate also reduces the need for phototherapy in healthy individuals. NouriShadkam et al. [18] showed that clofibrate was ineffective on TSB on the first day, but on the second day, it was effective in decreasing TSB. Eghbalian et al. [21] reported that clofibrate was an effective supplementary drug in neonatal hyperbilirubinemia, leading to decreased TSB levels and a shortened phototherapy duration in premature neonates. Sharafi et al. [8] showed that clofibrate was effective for outpatients with neonatal hyperbilirubinemia undergoing phototherapy at home.
Discussion
- According to the results of this study, administration of clofibrate decreased TSB, especially from the second day onwards, and also reduced hospitalization time, hospital costs, and hospitalization-associated complications.
- Larger randomized controlled trials (complying with all principles of study design) along with longer follow-up and consideration of hemolytic diseases and blood transfusion are needed to further elucidate this issue.
- The reviewed studies showed that doses of 25–100 mg/kg and short‑term administration of clofibrate did not lead to any complications during the treatment and follow‑up periods. Lipids and unconjugated bilirubin can conjoin with each other and bind to albumin. Therefore, changes in bilirubin levels must be adjusted with consideration of alterations in the lipid profile. In this regard, lipids are among the most important macronutrients, playing necessary roles in cell growth and development in newborns; therefore, the long‑term administration of clofibrate could impair organ development and growth.
- In the reviewed studies, complications were mostly evaluated by clinical observations, which could be considered as a limitation of clinical studies. Thus, it is recommended to perform laboratory tests and biochemistry examinations (according to the side effects) in future studies to obtain more useful results.
- Limitations of the Study
- The full text of some studies was not available, and some information required for data analysis was incomplete.
Conclusion
-
Ethics Approval
This study was approved by the Institutional Review Board of Hamadan University of Medical Sciences (IR.UMSHA.REC.1400.364) and performed in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of this study.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
All data generated or analyzed during this study are included in this published article. Other data may be requested through the corresponding author.
-
Authors’ Contributions
Conceptualization: RR; Data curation: FE; Formal analysis: RR; Investigation: AHD; Methodology: RR; Project administration: RR; Resources: FE; Software: LK; Supervision: RR; Validation: LK; Visualization: HB; Writing-original draft: RR; Writing-review & editing: all authors.
-
Additional Contributions
Lotfollah Karimi (Hamedan University of Technology, Hamedan, Iran) provided statistical support.
Article information

| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 |
|---|---|---|---|---|---|---|---|
| Ahadi et al. [2] | Low | Low | Low | Low | Low | High | Unclear |
| Gholami et al. [25] | Low | High | Low | Low | Low | Low | Low |
| Mahyar et al. [12] | Low | Low | Low | Low | Low | Low | High |
| Kumar et al. [23] | Low | Low | Low | Low | Unclear | Low | Low |
| NouriShadkam et al. [18] | Low | Low | Low | Low | Low | Low | Unclear |
| Fallah et al. [6] | Low | Low | Low | Unclear | Low | Low | Low |
| Habibi et al. [16] | Low | Low | Low | High | Low | Low | Low |
| Alosy [24] | High | Low | Low | Low | Low | Low | Low |
| Kandharkar [27] | High | Low | Low | Low | Low | Low | Low |
| Sakha et al. [26] | Low | Low | Low | Low | Low | Low | High |
| Zahedpasha et al. [7] | Low | Low | Unclear | High | Low | Low | Low |
| Moslehi and Pishva [17] | Low | High | Low | Low | Low | Low | Low |
| Badeli et al. [1] | Low | Low | Low | Unclear | Low | Low | Low |
| Zahedpasha et al. [28] | Low | Unclear | Low | Low | High | Low | Low |
| Sharafi et al. [8] | Low | Low | Low | Unclear | Low | Low | Low |
| Mohammadzadeh et al. [29] | Low | Unclear | Low | Low | Low | High | Low |
| Mohammadzadeh et al. [11] | Low | High | Low | Low | Low | Low | High |
| Study | Year of publication | Type of study | Country | Sample size | No. of girls | No. of boys | Age (d) | weight (kg) | Dosage of clofibrate (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Ahadi et al. [2] | 2013 | Double-blind clinical trial | Iran | 30 | 16 | 14 | 2.09 | 2.5−4 | 100 |
| Gholami et al. [25] | 2019 | Randomized controlled clinical trial | Iran | 30 | 4.45 | 3.193−3.460 | 50 | ||
| Gholami et al. [25] | 2019 | Randomized controlled clinical trial | Iran | 30 | 4.45 | 3.193−3.460 | 100 | ||
| Mahyar et al. [12] | 2019 | Randomized clinical trial | Iran | 20 | 11 | 9 | 5 | 3.196 | 30 |
| Kumar et al. [23] | 2017 | Randomized controlled trial | India | 45 | 24 | 21 | 4 | 2.760 | 50 |
| NouriShadkam et al. [18] | 2016 | Double-blind randomized clinical trial | Iran | 41 | 21 | 20 | 6.41 | 3.490 | 100 |
| Fallah et al. [6] | 2012 | Parallel single-blind randomized clinical trial | Iran | 30 | 14 | 16 | 4.73 | 3.202 | 50 |
| Habibi et al. [16] | 2012 | Single-blind clinical trial | Iran | 26 | 11 | 15 | 3.31 | 3.057 | 100 |
| Alosy [24] | 2017 | Case-control study | Iraq | 100 | 100 | ||||
| Kandharkar [27] | 2019 | Randomized controlled trial | India | 32 | 16 | 16 | 3.1 | 2.845 | 100 |
| Sakha et al. [26] | 2009 | Double-blind, placebo-controlled, randomized trial | Iran | 35 | 15 | 20 | 6.2 | 2.330 | 100 |
| Zahedpasha et al. [7] | 2008 | Randomized clinical trial | Iran | 30 | 16 | 14 | 5.67 | 3.083−3.300 | 100 |
| Moslehi and Pishva [17] | 2007 | Clinical randomized controlled trial | Iran | 30 | 13 | 17 | 5.25 | 2.564 | 25 |
| Moslehi and Pishva [17] | 2007 | Clinical randomized controlled trial | Iran | 30 | 16 | 14 | 5.18 | 2.525 | 50 |
| Badeli et al. [1] | 2008 | Clinical trial | Iran | 45 | 19 | 26 | 5 | 3.190 | 100 |
| Zahedpasha et al. [28] | 2008 | Randomized clinical trial | Iran | 21 | 5.81 | 3.195−5.200 | 100 | ||
| Sharafi et al. [8] | 2010 | Clinical trial | Iran | 30 | 18 | 12 | 6.8 | 3.107 | 50 |
| Mohammadzadeh et al. [29] | 2009 | Randomized double-blind placebo-controlled trial | Iran | 30 | 18 | 12 | 8.7 | 2.186 | 100 |
| Mohammadzadeh et al. [11] | 2005 | Clinical controlled study | Iran | 30 | 10 | 20 | 9.8 | 3.257 | 100 |
- 1. Badeli HR, Sharafi R, Sajedi SA. The effect of clofibrate on neonatal hyperbilirubinemia in uncomplicated jaundice. Iran J Pediatr 2008;18:20−4.
- 2. Ahadi A, Mirzarahimi M, Ahmadabadi F, et al. Comparison of the efficacy of Clofibrate with phenobarbital in decreasing neonatal hyperbilirubinemia. Iran J Neonatol 2013;4:9−13.
- 3. Eghbalian F, Hasanpour-Dehkordi A, et al. The effects of clofibrate on neonatal jaundice: a systematic review. Int J Prev Med 2022;13:3. ArticlePubMedPMC
- 4. Slusher TM, Zamora TG, Appiah D, et al. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr Open 2017;1:e000105.ArticlePubMedPMC
- 5. Zahed Pasha Y, Alizadeh-Tabari S, Zahed Pasha E, et al. Etiology and therapeutic management of neonatal jaundice in Iran: a systematic review and meta-analysis. World J Pediatr 2020;16:480−93.ArticlePubMedPDF
- 6. Fallah R, Islami Z, Lotfi SR. Single dose of 50 mg/kg clofibrate in jaundice of healthy term neonates: randomised clinical trial of efficacy and safety. Indian J Pediatr 2012;79:194−7.ArticlePubMedPDF
- 7. Zahedpasha Y, Ahmadpour-Kacho M, Hajiahmadi M, et al. Effect of clofibrate in jaundiced full-term infants:a randomized clinical trial. Arch Iran Med 2007;10:349−53.PubMed
- 8. Sharafi R, Mortazavi Z, Sharafi S, et al. The effect of clofibrate on decreasing serum bilirubin in healthy term neonates under home phototherapy. Iran J Pediatr 2010;20:48−52.PubMedPMC
- 9. Olusanya BO, Kaplan M, Hansen TW. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health 2018;2:610−20.ArticlePubMed
- 10. Chee YY, Chung PH, Wong RM, et al. Jaundice in infants and children: causes, diagnosis, and management. Hong Kong Med J 2018;24:285−92.ArticlePubMed
- 11. Mohammadzadeh A, Farhat AS, Iranpour R. Effect of clofibrate in jaundiced term newborns. Indian J Pediatr 2005;72:123−6.ArticlePubMedPDF
- 12. Mahyar A, Mehrpisheh S, Khajeh B, et al. The effect of purgative manna and clofibrate on neonatal unconjugated hyperbilirubinemia. Acta Medica Iran 2019;57:368−73.ArticlePDF
- 13. De Regnier RA. Neonatal jaundice: more than a number. J Pediatr 2017;183:2−3.Article
- 14. Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med (Lond) 2017;78:699−704.ArticlePubMed
- 15. Das S, van Landeghem FK. Clinicopathological spectrum of bilirubin encephalopathy/kernicterus. Diagnostics (Basel) 2019;9:24. ArticlePubMedPMC
- 16. Habibi M, Mahyar A, Ayazi P, et al. The effect of clofibrate on hyperbilirubinemia of term neonates. Acta Med Iran 2012;50:21−5.PubMed
- 17. Moslehi MA, Pishva N. Determination of effect of low dose vs moderate dose clofibrate on decreasing serum bilirubin in healthy term neonates. Iran J Pediatr 2007;17:108−12.
- 18. NouriShadkam M, Mohammadi MJ, Nasiriani K. Evaluation of the effect of oral clofibrate intake on neonatal total serum bilirubin: a randomized clinical trial. Iran J Neonatol 2016;7:5−8.
- 19. Eghbalian F, Pourhossein A, Zandevakili H. Effect of clofibrate in non-hemolytic indirect hyperbiliru-binemia in full term neonates. Indian J Pediatr 2007;74:1003−6.ArticlePubMedPDF
- 20. Eghbalian F, Monsef F, Alam Ghomi N, et al. Effect of low versus moderate dose of Clofibrate on serum bilirubin in healthy term neonates with indirect hyperbilirubinemia. Iran J Med Sci 2013;38:349−50.PubMedPMC
- 21. Eghbalian F, Jenabi E, Hatami E, et al. Clofibrate in the treatment of the non-hemolytic hyperbilirubinemia in preterm neonates in Western Iran. Iran J Neonatol 2021;12:48−52.
- 22. Xiong T, Chen D, Duan Z, et al. Clofibrate for unconjugated hyperbilirubinemia in neonates: a systematic review. Indian Pediatr 2012;49:35−41.ArticlePubMedPDF
- 23. Kumar P, Adhisivam B, Vishnu Bhat B. Clofibrate as an adjunct to phototherapy for unconjugated hyperbilirubinemia in term neonates. Indian J Pediatr 2017;84:763−7.ArticlePubMedPDF
- 24. Alosy BD. Benefit of Clofibrate on indirect hyperbilirubinemia in newborn. Al-Mustansiriyah J Pharma Sci 2017;17:134−9.ArticlePDF
- 25. Gholami N, Hosseini R, Naseh A. Clofibrate for non-hemolytic icterus in term newborns. J Compr Pediatr 2019;10:e90011.
- 26. Sakha SH, Gharehbaghi MM, Rahbani ME. The effect of clofibrate with phototherapy in late pre-term newborns with non-hemolytic jaundice. Indian J Med Sci 2009;63:174−9.ArticlePubMed
- 27. Kandharkar V. Use of clofibrate unconjugated hyperbilirubinemia in neonates. Int J Med Sci Diagn Res 2019;3:126−9.
- 28. Zahedpasha Y, Ahmadpour-Kacho M, Hajiahmadi M, et al. Efficacy of clofibrate on severe neonatal jaundice associated with glucose-6-phosphate dehydrogenase deficiency (a randomized clinical trial). Southeast Asian J Trop Med Public Health 2008;39:557−61.PubMed
- 29. Mohammadzadeh A, Farhat AS, Amiri R, et al. Treatment effect of clofibrate in jaundiced low birth weight neonates. Int J Hematol Oncol 2009;19:100−5.
- 30. Caballero-Noguez B, Hernandez PS, Rodriguez BJ, et al. Chlorifibrate effect associated with phototherapy on bilirubin concentration in newly-born babies. Rev Mex Pediatr 2001;68:176−80.
- 31. Eghbalian F, Monsef F, Alam Ghomi N, et al. Effect of low versus moderate dose of clofibrate on serum bilirubin in healthy term neonates with indirect hyperbilirubinemia. Iran J Med Sci 2013;38:349−50.PubMedPMC
- 32. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev 2012;(12):CD009017. ArticlePubMedPMC
References
Figure & Data
References
Citations

- Figure
- Related articles
-
- Predictors of outcomes 3 to 12 months after traumatic brain injury: a systematic review and meta-analysis
- Global prevalence of enterobiasis in young children over the past 20 years: a systematic review and meta-analysis
- Effects of medication adherence interventions for older adults with chronic illnesses: a systematic review and meta-analysis
- Associations of pre-existing cardiovascular morbidity with severity and the fatality rate in COVID-19 patients: a systematic review and meta-analysis
- Worldwide prevalence of fungal coinfections among COVID-19 patients: a comprehensive systematic review and meta-analysis


 PubReader
PubReader Cite
Cite