Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 12(1); 2021 > Article
-
Original Article
A Healthcare-Associated Outbreak of HCV Genotype 2a at a Clinic in Seoul - Siwon Choia, Hyerim Leeb, Hyungmin Leec, Yoon-Seok Chungd
-
Osong Public Health and Research Perspectives 2021;12(1):3-12.
DOI: https://doi.org/10.24171/j.phrp.2021.12.1.02
Published online: February 23, 2021
aJeju Branch Office, Honam Regional Center for Disease and Prevention, Korea Disease Control and Prevention Agecy, Jeju, Korea
bDivision of Immunization, Bureau of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agecy, Cheongju, Korea
cKorea Disease Control and Prevention Agecy, Cheongju, Korea
dDivision of Infectious Disease Diagnosis Control, Honam Regional Center for Disease and Prevention, Korea Disease Control and Prevention Agecy, Gwangju, Korea
- *Corresponding author: Siwon Choi, Jeju Branch Office, Honam Regional Center for Disease and Prevention, Korea Disease Control and Prevention Agecy, Jeju, Korea, E-mail: siwon1221@korea.kr
©2021 Korea Disease Control and Prevention Agency
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 5,512 Views
- 235 Download
Abstract
-
Objectives
- An epidemiological investigation was conducted into a hepatitis C virus (HCV) outbreak at an outpatients clinic in Seoul (2011–2012). The aim of the study was to analyze the scale of infection, identify the source of infection, and route of transmission to prevent hepatitis C transmission in the future.
-
Methods
- A retrospective study of the outpatients and health care workers (n = 7,285) in the target outpatient clinic during 2011–2012 was conducted. The history of the study population infection with hepatitis C, electronic medical records, field visits, and health care worker interviews were examined for the period between March 1st, 2006 and March 25th, 2016. The blood samples were collected and tested for anti-HCV antibodies, HCV RNA and HCV gene in 2016.
-
Results
- The rate of anti-HCV positive results was 4.4% in the study population. The risk factors associated with an anti-HCV positive result were ≥ 10 clinic visits, and receiving an invasive procedure including a nerve block and a block of the peripheral branch of the spinal nerve (p < 0.05). There were 112 HCV RNA positive cases out of 320 anti-HCV positive test result cases, amongst which 100 cases had the dominant HCV genotype 2a which formed either 1 cluster (n = 56) or 2 clusters (n = 25). This result indicated exposure to a high-association infection source.
-
Conclusion
- Anti-HCV antibodies and genotypic analysis showed an epidemiological association between the outbreak of HCV and invasive procedures performed (2011–2012) at an outpatients clinic in Seoul.
- Hepatitis C is an acute or chronic liver disease caused by hepatitis C virus (HCV). The incubation period is around 2 weeks to 6 months, and 80% of patients are asymptomatic after the initial infection. However, some patients present with fever, fatigue, reduced appetite, nausea, vomiting, upper abdominal pain, and jaundice. Approximately 15–45% of infected individuals recover naturally (without treatment) within 6 months, but the remaining 60–80% of individuals develop chronic hepatitis, of whom 15–30% show a risk of transition to liver cirrhosis within 20 years [1].
- According to the World Health Organization, in 2015 around 71 million people worldwide are living with chronic hepatitis C infection, which is approximately 1% of the global population. Moreover, it is estimated that 399,000 people die each year from cirrhosis of the liver or liver cancer caused by hepatitis C infection [1].
- In Korea, data from the 2016 Korea National Health and Nutrition Examination Survey (KNHANES) revealed that the rate of an anti-HCV positive result estimated for the period of 2012–2016 was 0.6% in males and 0.7% in females aged ≥ 10 years, and the rate showed an increasing trend with age [2].
- Hepatitis C has been designated as a national notifiable infectious disease in Korea since 2001, with several medical institutions reporting sentinel surveillance. However, since June 3rd, 2017, the disease has been designated as a Group 3 infectious disease, for which mandatory surveillance is required. According to the 2017 Infectious Diseases Surveillance Yearbook of the Korea Centers for Disease Prevention and Control (KCDC), reports of sentinel surveillance of hepatitis C showed a decreasing trend after a peak of 6,407 cases in 2008, followed by an increase to 3,793 in 2013, and 6,283 in 2016. After the introduction of mandatory surveillance, the report revealed 6,396 cases from June 3rd to December 31st, 2017 and 10,811 in 2018 [3,4].
- Hepatitis C is a blood-borne infectious disease, and its transmission route is via direct exposure through transfusion of HCV-contaminated blood or drugs, organ transplantation, unsafe use of invasive tools such as syringes, unsafe procedures, or wounds caused by a syringe or needle. Other known transmission routes are via sexual intercourse and vertical transmission from pregnant mothers to fetuses [5]. The World Health Organization reported that the main cause of hepatitis C was unsafe medical procedures and injections, which accounted for most new incidences (1.75 million cases) in 2015 [6]. In Korea, an epidemiological investigation on a hepatitis C outbreak was conducted in 2015 at a clinic in Seoul, with the presumed cause being the reuse of disposable syringes. In 2016, another epidemiological investigation on a hepatitis C outbreak was conducted at a clinic in Wonju, Kangwon-do, where invasive procedures were deemed to be the potential cause [7].
- A hepatitis C outbreak at a clinic in Seoul (2011–2012) was identified through a report of suspected reuse of disposable syringes and big data analysis by the National Health Insurance Service in 2016. In 2016, the KCDC and the Seoul city conducted an epidemiological investigation on the hepatitis C outbreak in the clinic in Seoul. This study aimed to report the results of the investigation and epidemiological characteristics of the hepatitis C outbreak.
Introduction
- 1. Study population and study design
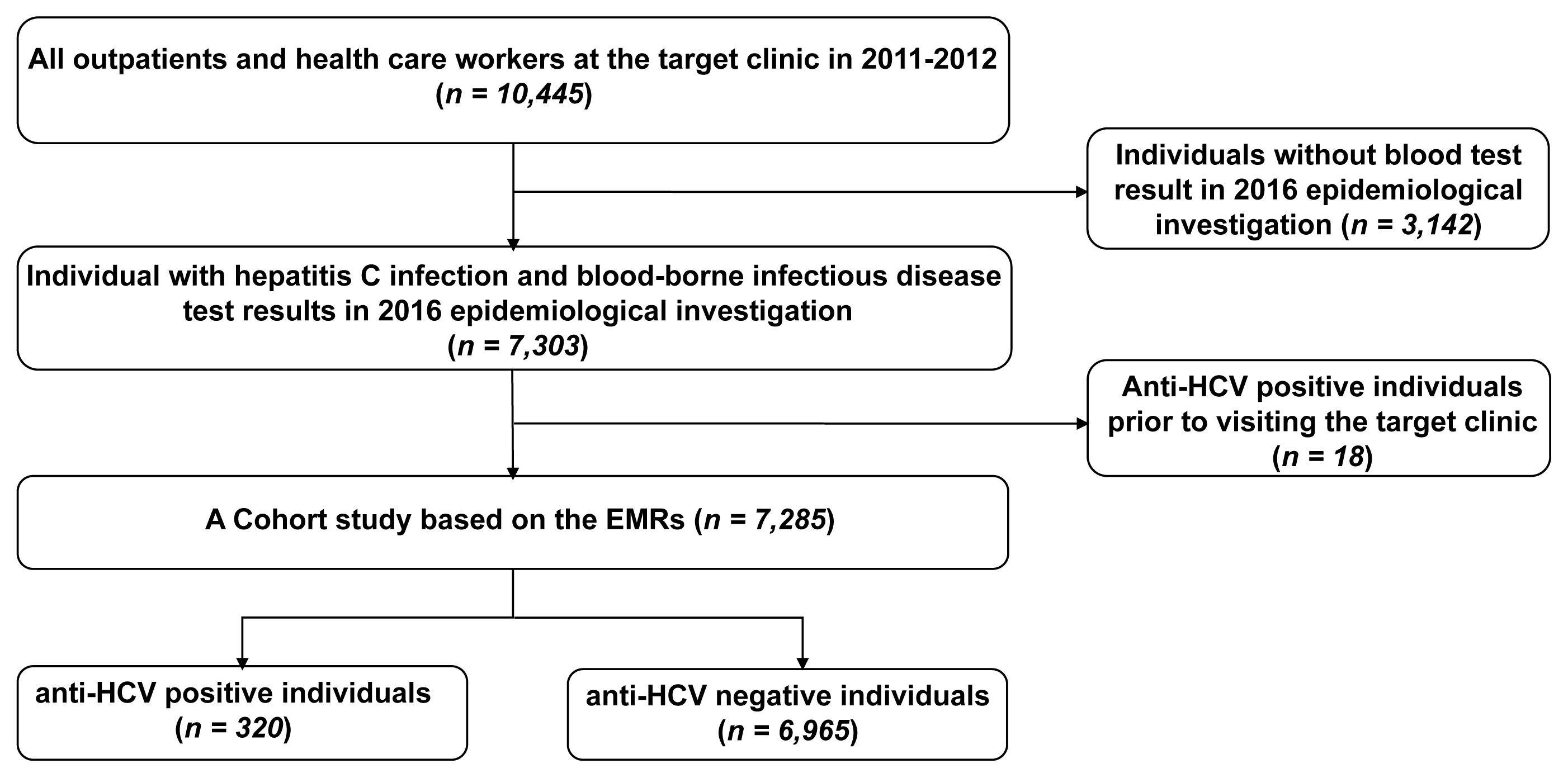
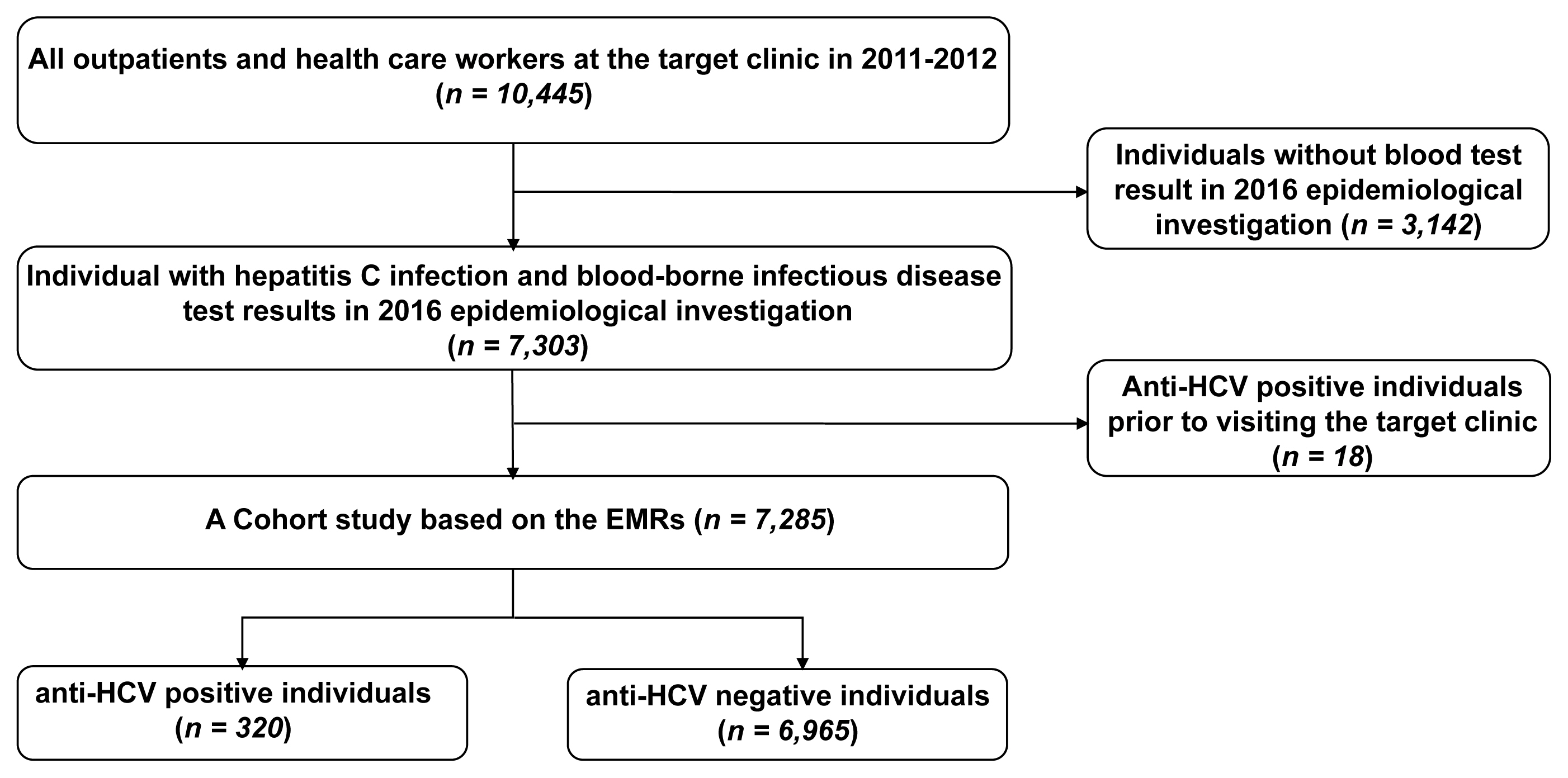
- Among all outpatients at the clinic during the period of 2011–2012, 7,303 individuals were tested for hepatitis C during the 2016 epidemiological investigation. Of these outpatients, 7,285 were selected for retrospective cohort analysis, after excluding 18 individuals with a history of an anti-HCV positive test result before visiting the clinic.
- 2. Data collection
- To examine the study participant history of infection with hepatitis C, the Health Insurance Review and Assessment Service was requested to provide hepatitis C test results of 2011–2012 outpatients at the target clinic for the period between March 1st, 2006 and March 25th, 2016. The anti-HCV tests, HCV RNA tests, and genotype tests over the past 10 years were examined. Thereafter, the test results were checked through the city/province of the hospital and clinic at which the tests had been performed, and the records on blood donation and mortality were examined.
- From the electronic medical records of the target clinic for the period 2011–2012, information on the outpatients’ sex, age, treatment, date of visit, and frequency of visit was examined.
- On August 18th and 19th, 2016, the KCDC conducted a field investigation. All health care workers, including doctors and certified nursing assistants were interviewed, and the infection control status in the doctor’s office and injection room was examined. On March 24th and 25th, 2016, officials from the local public health center visited the target clinic to collect 10 environmental samples (lidocaine ampules, syringe needles, and NaCl solution in syringes) for the basic investigation. The HCV RNA was not detected in the test samples. Environmental samples were not collected during the field investigation by the KCDC in 2016-because of time difference (with suspected risk factors in 2011–2012).
- 3. Laboratory tests
- Between August 25th and December 16th, 2016, hepatitis C tests were performed on samples from the target clinic taken from outpatients and health care workers in 2011–2012. Anti-HCV antibodies were tested. Then, the samples which were HCV RNA positive were used for HCV genotype testing and gene sequencing analysis. For the HCV genotype test, the Abbott HCV genotype kit was used, and for gene sequencing analysis, Core-E2 gene sequencing was carried out for analyses of the genotype and transmission groups.
- RNA was extracted from 140 μL of patient serum with the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), then cDNA was synthesized with the SuperScrip 3 First-Strand Synthesis System (Thermo Scientific, Grand Island, NY, USA), following the manufacturer’s instructions. For the HCV core-E2 region, the first PCR was performed with HCV134F (5′-AGA GCC ATA GTG GTC TGC GGA A-3′) and HCV1987R (5′-TTC ATC CAB GTR CAR CCR AAC C-3′), and the nested PCR with HCV278F (5′-GCC TTG TGG TAC TGC CTG ATA G-3′) and HCV1791R (5′-GSG TAR TGC CAG CAR TAN GG-3′), by pre-denaturation at 95°C for 1 minute, and 40 cycles of denaturation at 95°C for 20 seconds, and extension at 68°C for 1 minute using PrimeSTAR Max DNA Polymerase (Takara Bio, Shiga, Japan). For the NS5B region, the first PCR was performed with HCV8250F (5′-TTC TCR TAT GAY ACC CGC TGY TTT GA-3′) and HCV8616R (5′-TAC CTV GTC ATA GCC TCC GTG AA-3′), and the nested PCR with NS5B-nested-F (5′-CGC TGC TTT GAC TCT ACA GTC ACT G-3′) and NS5B-nested-R (5′-TCT CAG GCT TGC TGC ATC CTC-3′), under the PCR conditions described for the core-E2 region, except the extension was performed at 68°C for 30 seconds.
- PCR amplicons were sequenced on the ABI BigDye sequencer (Applied Biosystems, Foster City, CA, USA), and the sequences were assembled using DNA Baser Sequence Assembler v.4.20 (http://www.dnabaser.com/download/DNA-Baser-sequence-assembler/). To determine the HCV subtypes, the Los Alamos HCV database was used (http://hcv.lanl.gov/) and the Oxford HCV Subtyping Tool (http://bioafrica.net/rega-genotype/html/subtypinghcv/html).
- The assembled sequences were aligned based on the NS5B and core-E2 regions in the CLC Main Workbench (Qiagen Bioinformatics, Redwood City, CA, USA) using ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Phylogenetic analysis was initially performed with MEGA 6.06 (http://www.megasoftware.net/), and created an alignment of 1,367 nucleotides. The genetic subtypes and potential transmission clusters from the time-stamped sequence dataset were first deduced by neighbor joining tree reconstruction, using MEGA Version 6.06, based on the GTR G+I parameter Model. The robustness of the transmission clusters was further tested by the more rigorous maximum likelihood inference, implemented in MEGA Version 6.06, using gamma distribution with discrete gamma categories. The reliability of the branching orders was assessed by bootstrap analysis of 1,000 replicates. The most appropriate nucleotide substitution model was determined using FindModel, a web implementation of MEGA Version 6.0.6.
- 4. Statistical analysis
- The comparison of general characteristics between the whole study population and the participant’s with an anti-HCV positive test result was presented as frequency and percentage. To analyze the difference between the rate of anti-HCV positive test results between the group exposed to the risk factors and the group without exposure to the risk factors a relative risk ratio was calculated, and the Chi-square test was performed. To determine the inter-group, independent association amongst variables, multivariate logistic regression analysis was performed. All data were analyzed using the Statistical Package for Social Science, SPSS (verson 18.0; SPSS Inc., Chicago, IL, USA), and for all analyses, p < 0.05 indicated statistical significance.
Materials and Methods
3.1. RNA extraction and polymerase chain reaction (PCR) amplification
3.2. Sequencing and genotyping
3.3. Phylogenetic analysis
- 1. Case enrollment
- Among the 7,303 individuals who underwent hepatitis C tests during the epidemiological investigation in 2016, those with a history of an anti-HCV positive test result before visiting the target clinic (18 individuals) were excluded, and 7,285 were included in the analysis. The number of individuals with an anti-HCV positive test result was 320, whereas the number of anti-HCV with a negative test result was 6,965 (Figure 1).
- 2. General characteristics
- Among the 7,285-study population, there were 2,312 (31.7%) males and 4,973 (68.3%) females, and the group ≥ 65 years of age had the largest number of study population, at 3,556 (48.8%). Among the 320 (4.4% of the study population) individuals who had an anti-HCV positive test result, there were 83 (25.9%) males and 237 (74.1%) females, and the group ≥ 65 years of age had the largest number of cases 209 (65.3%; Table 1).
- 3. Characteristics of the risk factors
- From January 1st, 2011 to December 31st, 2012, the mean frequency of visits to the target clinic was 8.6 times, and 4,234 (58.1%) individuals visited the target clinic less than 5 times. However, 157 (49.1%) individuals with an anti-HCV positive test visited the clinic 10 times or more (Table 2) with an average of 19.1 times.
- Among the 7,285-study population, 6,696 (91.9%) had a history of invasive procedures more than once. This was similar to individuals with an anti-HCV positive test 316 (98.8%). In terms of the specific procedures most individuals 5,146 (70.6%) had received a block of the peripheral branch of the spinal nerve this was 320 (92.8%) individuals for those with an anti-HCV positive test (Table 2).
- 4. Analysis of the risk factors
- Univariate analysis revealed that the relative risk ratio for hepatitis C infection was significantly high for females (1.34 [95% CI: 1.04–1.73]), for the group ≥ 65 years (5.72 [95% CI: 3.37–9.69]), for invasive procedures (7.24 [95% CI: 2.69–19.49]), and for blocks to the peripheral branch of the spinal nerve [5.64 [95% CI: 3.68–8.64]; Table 3).
- Multivariate logistic regression analysis showed that the risk factors with an independent influence in individuals with an anti-HCV positive test result were aged ≥ 65 years (OR 2.15, 95% CI: 1.23–3.74), having ≥ 10 visits to the clinic (OR 4.22, 95% CI: 3.12–5.72), and a medical history of having received a cranial nerve block (OR 1.71, 95% CI: 1.29–2.26) or block of the peripheral branch of the spinal nerve (OR 2.14, 95% CI: 1.32–3.47; Table 4).
- 5. Field investigation
- During the period from 2011 to 2012, the health care workers at the target clinic comprised of 4 medical specialists, 2 certified nursing assistants, 1 physical therapist, and 2 other employees in the Departments of Anesthesiology, General Surgery, Internal Medicine, Dermatology, and Orthopedic Surgery. On the day of the field investigation, 2 medical specialists were performing the medical treatment using 2 consultation rooms and 1 treatment room.
- In the interview with a healthcare worker to identify the risk of infection during the invasive procedure, a specialist stated that a certified nursing assistant had been observed using 500 cc of normal saline multiple times during drug preparation for the injection, and that although the main steps of the procedure were performed by the specialist, certain steps were performed by the certified nursing assistants.
- Of note, the target clinic was not equipped with a sterilizer. All 4 specialists were unaware of the kind of sterilizer or detailed sterilization method required prior to the invasive procedure, and a certified nursing assistant stated that UV disinfection was a suitable method for sterilization, thereby confirming the absence of any equipment for sterilization at the clinic.
- 6. Laboratory test results
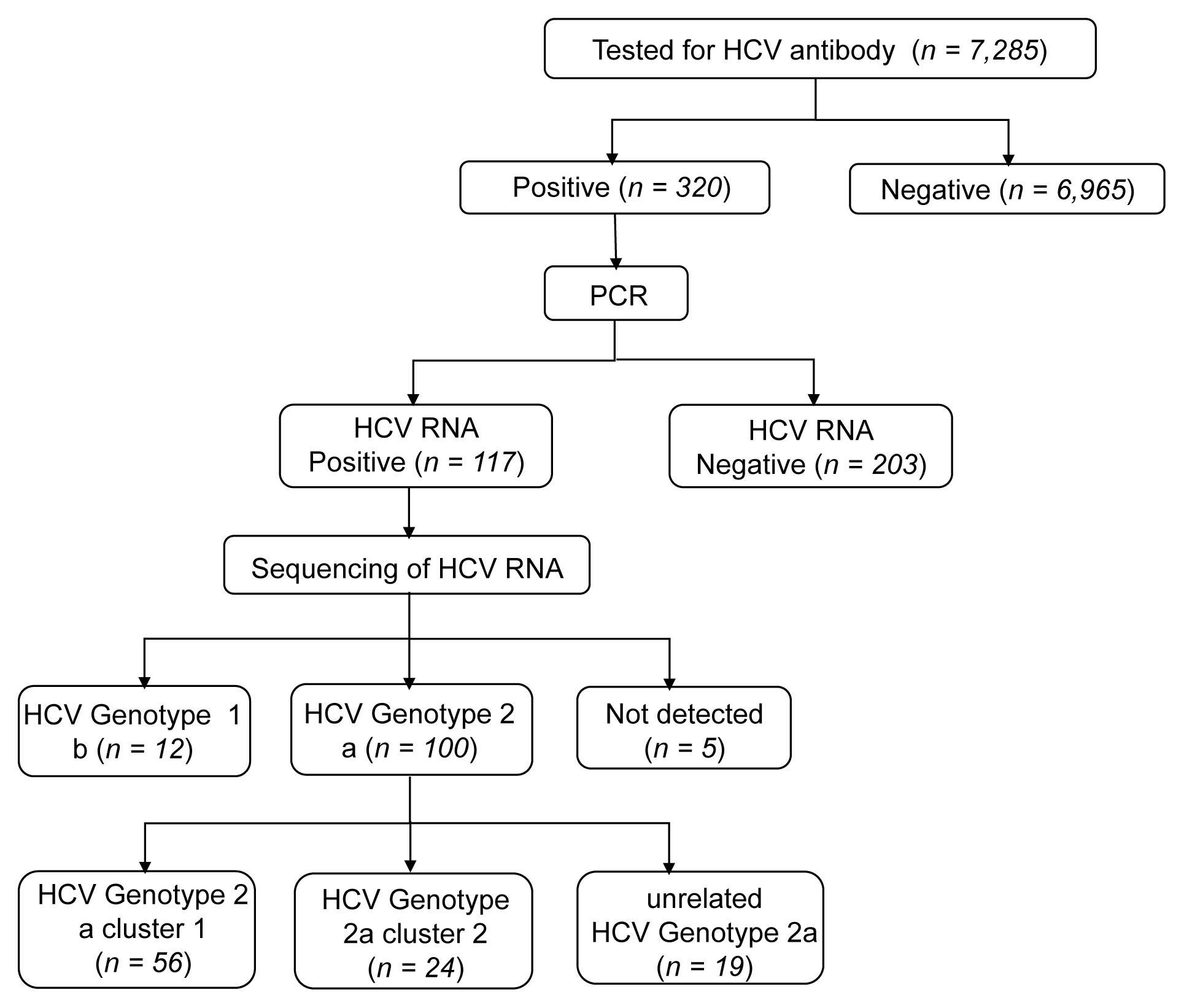
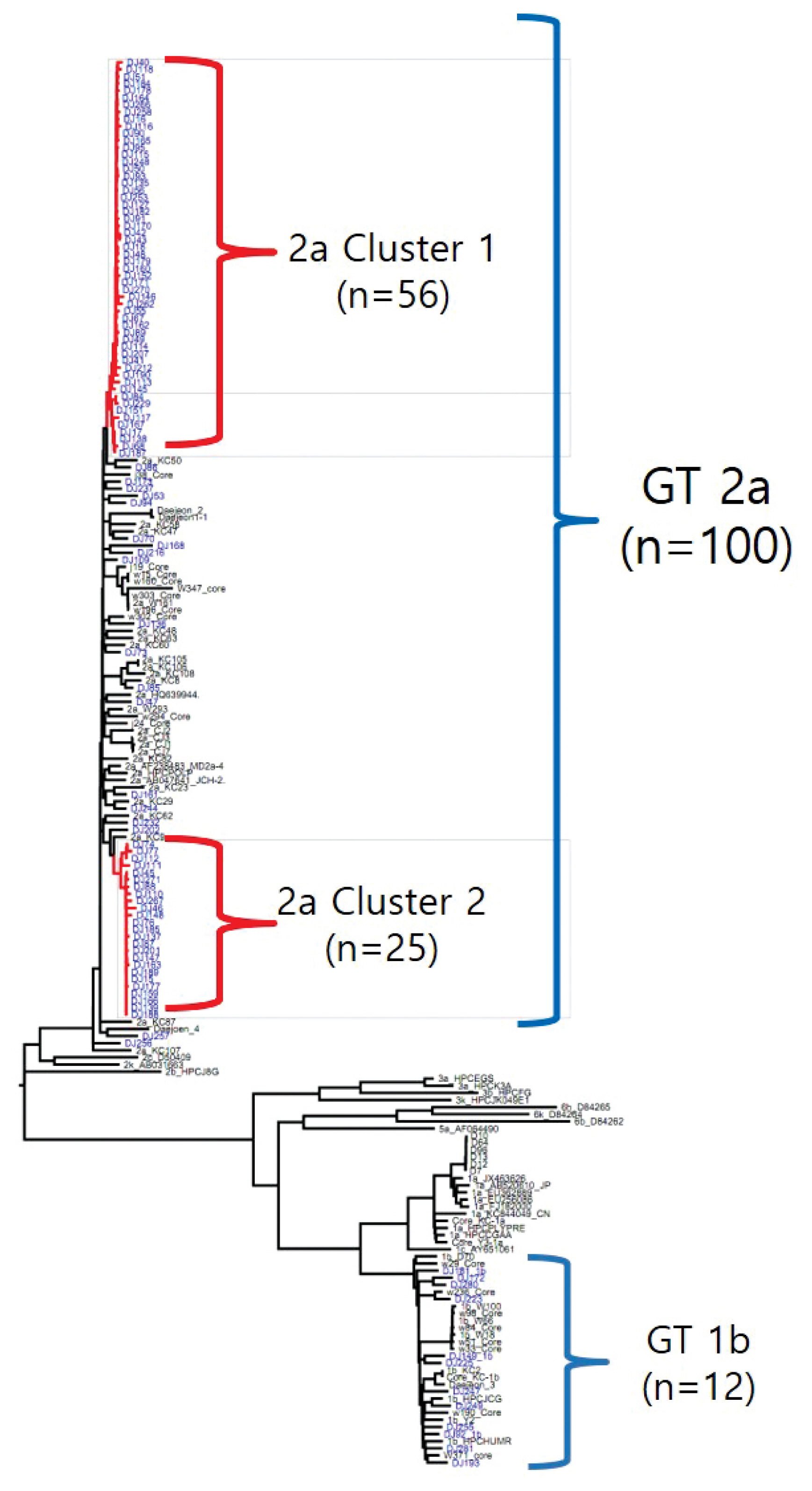
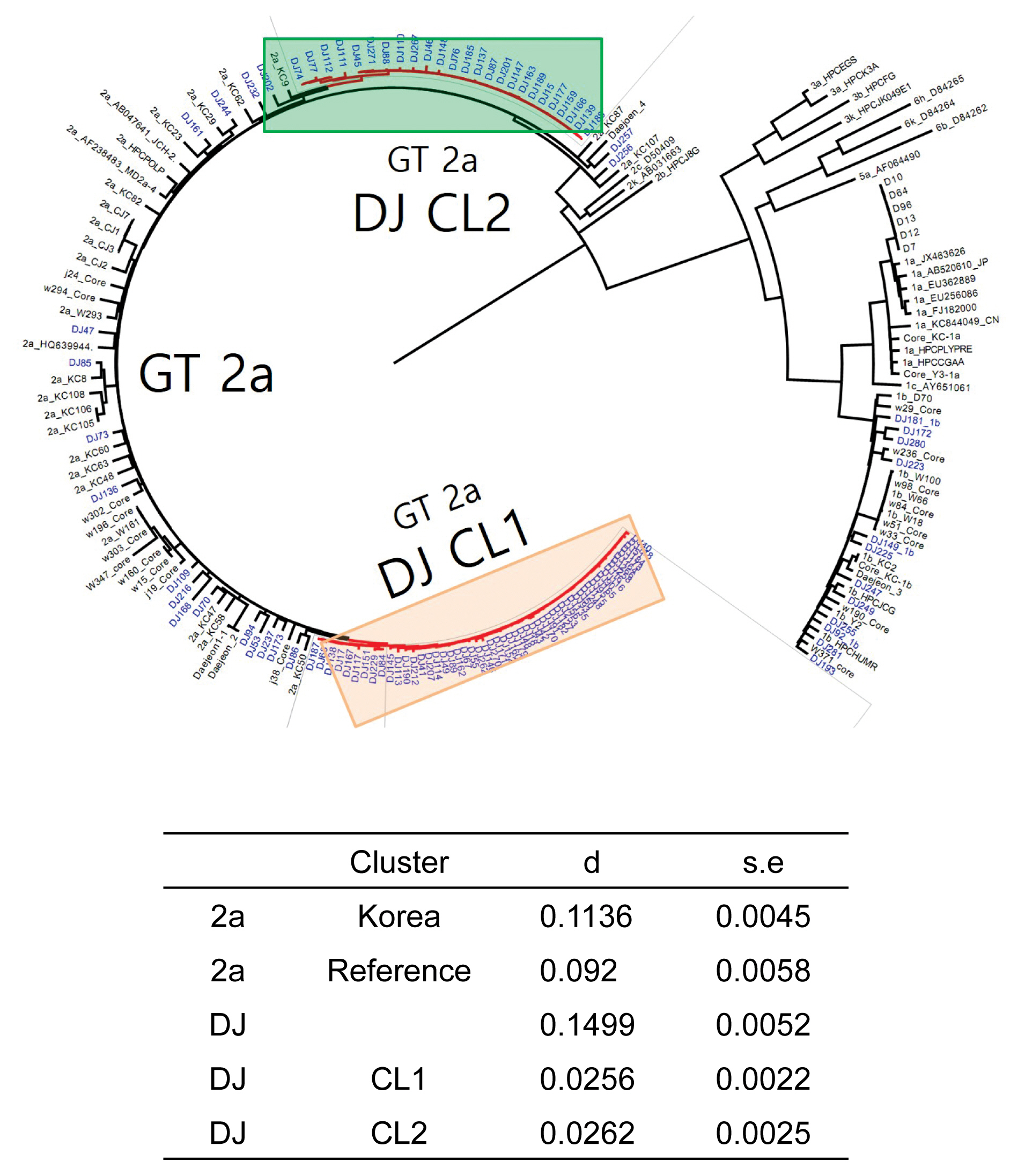
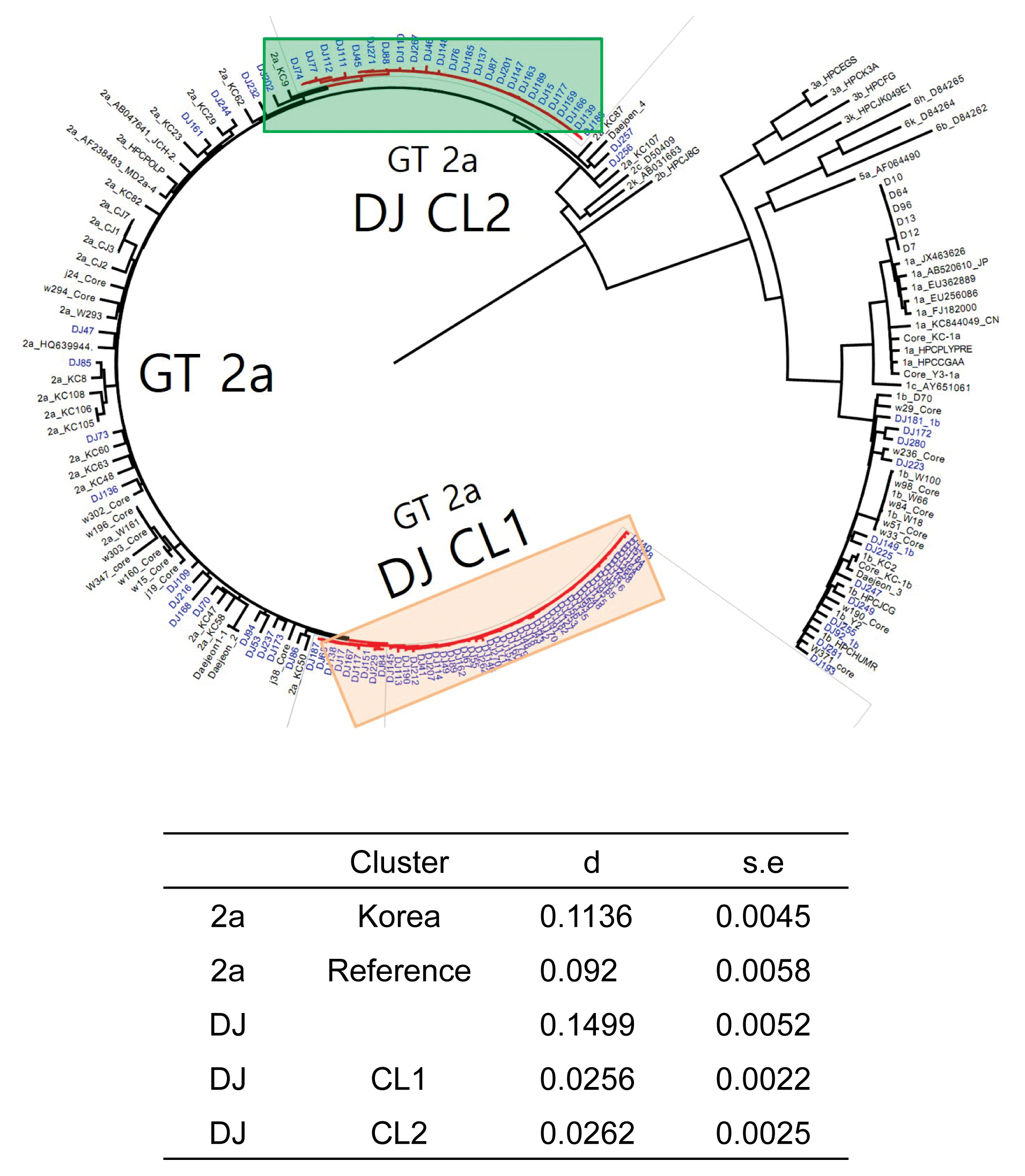
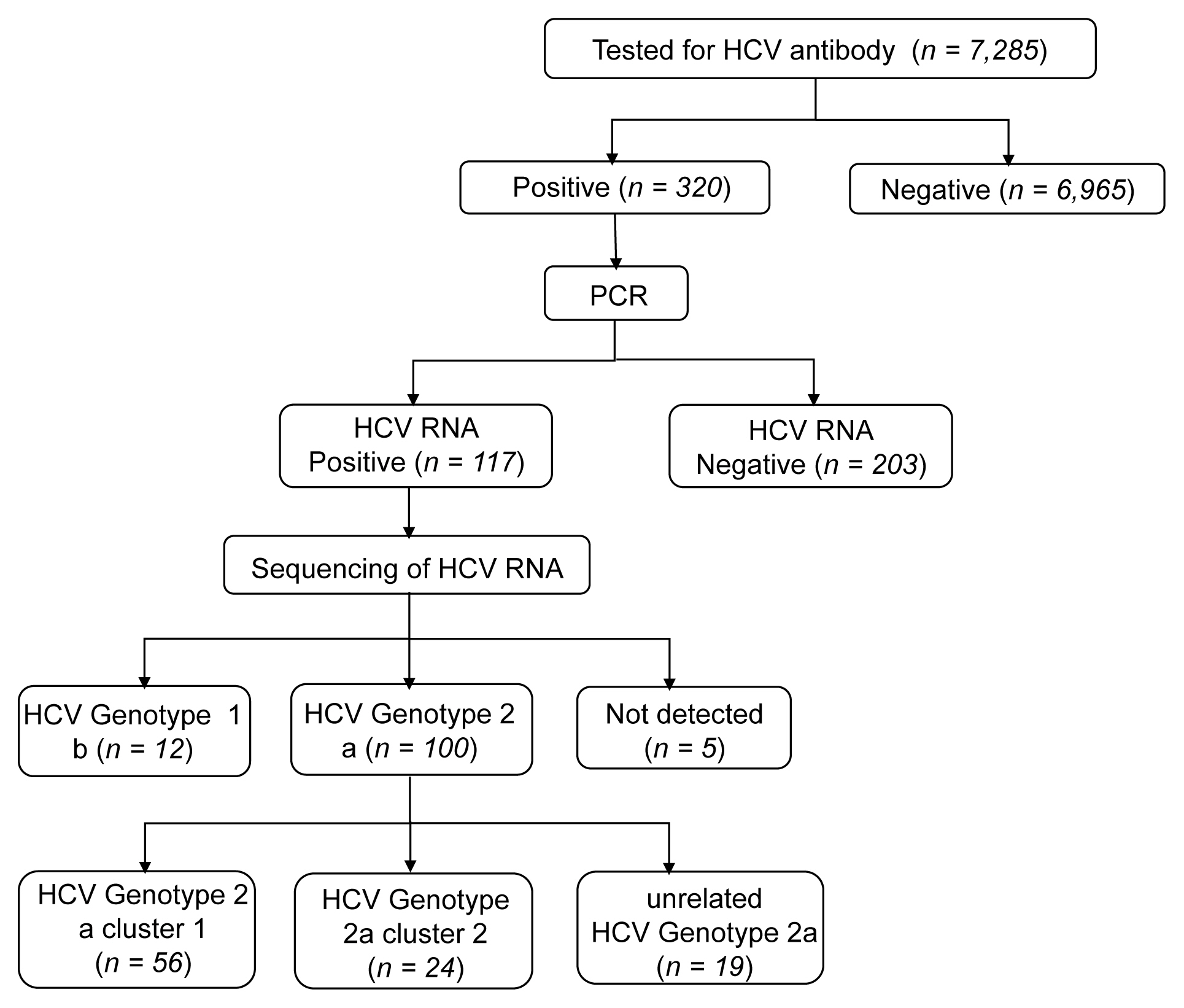
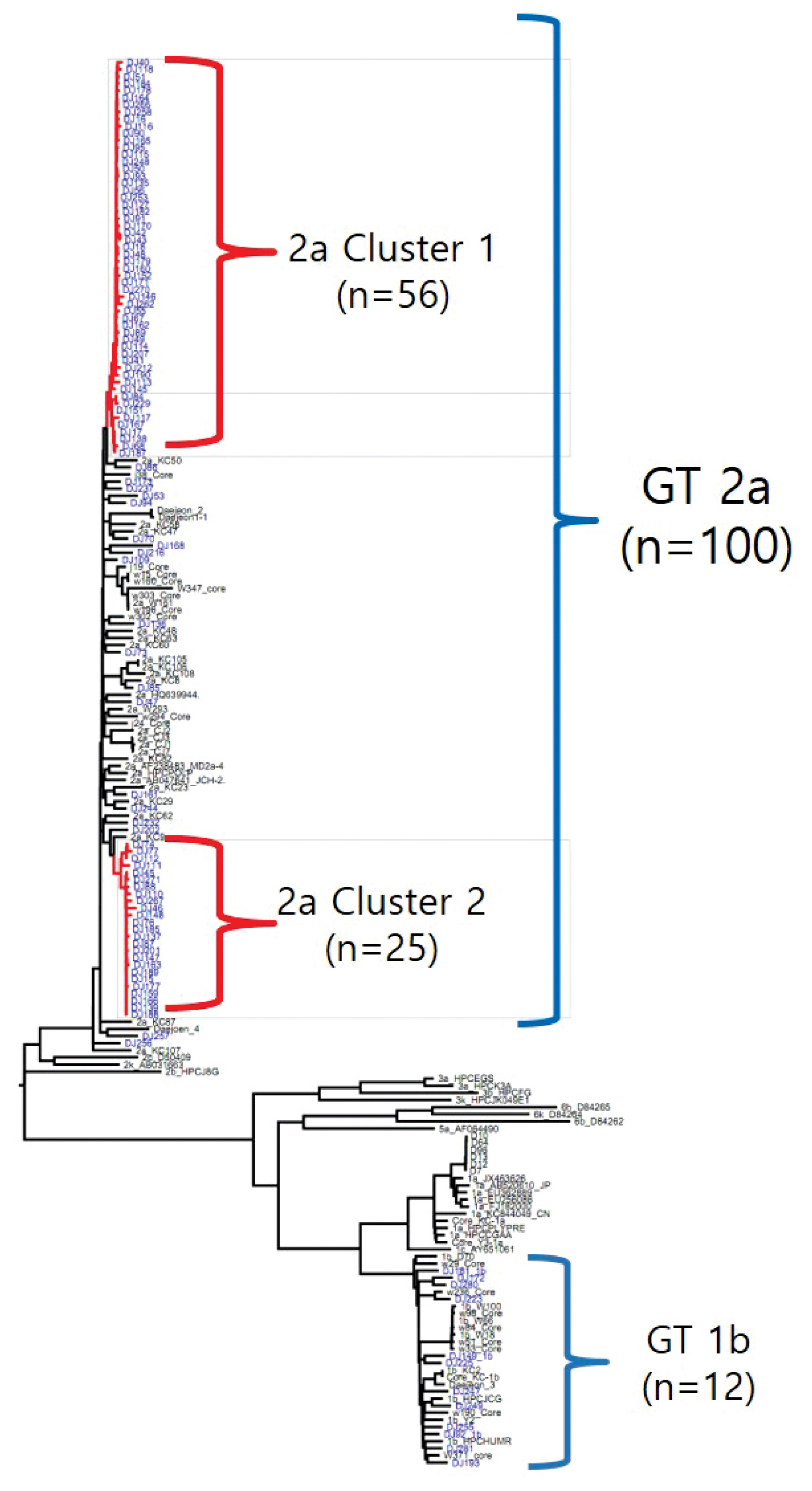
- The rate of anti-HCV positive results was 4.4% in the study population. Analysis of the genotyping of the core-E2 gene sequence in the hepatitis C transmission group revealed 112 HCV RNA positive cases out of 320 anti-HCV positive test result cases, amongst which 100 cases had genotype 2a and 12 cases had genotype 1b. The 100 cases with genotype 2a were segregated into 2 clusters: cluster 1 (n = 56) and cluster 2 (n = 25), each presumed to have been exposed to a high-association infection source. There were 19 cases of genotype 2a samples that did not belong to either cluster 1 or 2 (Figures 2-4).
Results
- The epidemiological investigation of the hepatitis C outbreak at a clinic in Seoul, showed 4.4% of individuals had an anti-HCV positive test, and 48% of these individuals were in the age group ≥ 65 years. The hepatitis C infection rate observed was ≥ 4 times higher than the rate reported for the age group 60–69 year-olds (males: 1.1%, females: 1.0%) who participated in the 2012–2016 Korea National Health and Nutrition Examination Survey (KNHANES) [2].
- Significant risk factors were associated with the frequency of clinic visits and the history of invasive procedures, especially those involving nerve blocks and a block of the peripheral branch of the spinal nerve. The transmission route of hepatitis C was thus determined to be via a specific invasive procedure that was conducted at the clinic. The Global Burden of Disease 2000, reported that the main source of infection in hepatitis C cases was unsafe use of medical syringes, with 30% of injections containing previously used substances, and 40% due to medical syringes being reused without cleaning and sterilization [8]. A report on healthcare-associated hepatitis C outbreaks in the U.S. during the period between 2008 and 2017, showed 295 hepatitis C patients in 38 outbreaks, but the number of people notified for for hepatitis C screening exceeded 100,000. In this instance, the hepatitis C outbreak was reported to have been caused mainly by drug contamination related to syringe reuse, hemodialysis, and unsafe protocols of use of medical injections by the health care workers in outpatient and hemodialysis facilities [9].
- In Korea blood transfusions were a main source of hepatitis C infection until 1991, after which blood screening for HCV began, and since 2005 no related cases have been reported [10,11]. A study of 1,137 HCV patients conducted in 5 university hospitals in Korea between 2007–2011 showed that the independent risk factors for HCV infection included abuse of intravenously injected drugs, wounds caused by needle puncture, blood transfusion received before 1995, tattooing, and age [12]. The risk for contracting HCV infection via subcutaneous exposure (such as puncture with a contaminated needle tip) has been reported in 2012 as 0.92% in Korea [13]. Healthcare-associated hepatitis C outbreaks in Korea have been caused by reusing contaminated syringes and performing specific invasive procedures (2015 outbreak at a clinic in Seoul and the 2016 outbreak at a clinic in Wonju) [7].
- Among the 112 people for whom HCV gene sequencing analysis was performed in the current study, genotype 2a formed 2 clusters, cluster 1 (n = 56) and cluster 2 (n = 25), thus indicating a probable genetic association. While HCV has 6 genotypes with over 70 subtypes, the 2a genotype is very common in Korea. Types 1, 2, and 3 are widely distributed across the world [14,15]. The most common HCV genotypes in Korea are 1b (45–90%) and 2a (26–51%), and the proportion of other genotypes (1a, 2b, 3, 4, and 6) is less [16,17]. In the hepatitis C outbreak at the clinic in Wonju, cluster formation was observed for the 2a (n = 58) and 1b (n = 90) genotypes [7].
- Among the 320 cases of an anti-HCV positive test result, there were 147 participants that were not recognized through hepatitis C PCR tests during epidemiological investigation. Epidemiological investigation will contribute to the early detection and treatment of hepatitis C in patients.
- There are limitations to this study firstly, among the 10,445 outpatients over the period 2011–2012, only 7,303 participated in the study and underwent testing for hepatitis C. Furthermore, analysis was performed with 7,285 (69.7%) outpatients after the exclusion of those with a history of an anti-HCV positive test result prior to visiting the target clinic. Had the rate of hepatitis C testing been higher, the size of the hepatitis C outbreak could have been more accurately determined. In addition, the hepatitis C infected patients among the non-tested individuals could have formed other clusters, the potential association between cases outside the sequenced clusters, and the outbreak, cannot be completely ruled out. Secondly, an inherent limitation of a cross-sectional study is the time difference between the risk assessment period (2011–2012 in this case) and the time period of the epidemiological investigation (2016). This could have prevented accurate collection of data regarding the onset of symptoms and the procedures undergone by patients. These parameters were thus excluded from the analysis, despite a basic survey being carried out on the participants who received a blood test or antibodies against hepatitis C in the 2016 epidemiological investigation. Since the history of procedures was examined according to the procedure name using only the electronic medical records, the data of procedures in the charges by insurance would not show procedures that were not covered. Had there been no time difference (as mentioned earlier), procedure data and onset of symptoms in hepatitis C positive patients would have been available for use. It would have provided evidence to identify the presumed source of infection and other infection risk factors. Thirdly, the time difference meant that a certain proportion of hepatitis C positive patients had been treated or had naturally recovered, thus leading to the sequencing analysis being focused only on the patients who still tested positive for the HCV RNA. Fourthly, the large number of participants prevented in depth analysis of other risk factors, such as a patient’s history of using a hospital other than the target clinic. Therefore, the other clinic risk factors of hepatitis C infection could not be analyzed. Fifthly, although staff at the target medical institution stated that normal saline had been used multiple times in drug preparation for the injection, this, as well as the syringe contamination, could not be verified. Hence, the accurate transmission route for the infection could not be identified.
- Despite these limitations, this study described the general characteristics of a large-scale hepatitis C outbreak at a clinic in Korea, and identified a genetic association based on the results of blood tests and a history of invasive procedures, as risk factors for hepatitis C infection. The findings of the current study are significant at the national level because they provide evidence for establishing preventive measures against hepatitis C. For the prevention of healthcare-associated infections, medical institutions should ensure that health care workers follow the processes of asepsis and hand hygiene, while complying with the recommendations for the injection to prevent infections [18]. In addition, to prevent the transmission of infectious diseases within a medical institution, there should be strict control over the environment, with continuous monitoring and surveillance based on thorough understanding of the infection control guidelines regarding the insert devices and the medical supplies. Further studies should focus on the incidence of hepatitis C in Korea, and the risk factors as well as the epidemiological investigation of the transmission of healthcare-associated hepatitis C.
Discussion
- Based on the data of the epidemiological investigation conducted by the KCDC in 2016 for the hepatitis C outbreak at a clinic in Seoul 2011–2012, the scale of infection was 4.4%, the common source of infection was HVC genotype 2a, and the route of transmission was invasive procedures (including a nerve block and a block of the peripheral branch of the spinal nerve).
Conclusion
-
Acknowledgements
- We express our deepest gratitude to all the employees of the relevant ministries, municipal governments, and community health centers who are working hard to prevent, and manage the hepatitis C outbreak investigation. We would also like to thank the Department of Infectious Disease Control in Dongjak-gu and Seoul.
Acknowledgments
-
Conflicts of Interest
The authors have no conflicts of interest to declare for this report.
Article information
| Variables | Study population | Anti-HCV positive |
|---|---|---|
| Total | 7,285 (100.0) | 320 (100.0) |
| Sex | ||
| Male | 2,312 (31.7) | 83 (25.9) |
| Female | 4,973 (68.3) | 237 (74.1) |
| Age (y) | ||
| Average [n (SD)] | 61.2 (15.4) | 67.3 (10.3) |
| < 50 | 1,388 (19.1) | 15 (4.7) |
| 50–64 | 2,341 (32.1) | 96 (30.0) |
| ≥ 65 | 3,556 (48.8) | 209 (65.3) |
| Hepatitis C infection | ||
| Previous anti-HCV positive test result* (as of March 31st, 2016) | 194 (2.7) | 173 (54.1) |
| Risk factors | Study population | Anti-HCV positive |
|---|---|---|
| Total | 7,285 (100.0) | 320 (100.0) |
| No. visits (2011–2012) | ||
| Average [n (SD)] | 8.6 (18.2) | 19.1 (31.1) |
| < 5 | 4,234 (58.1) | 74 (23.1) |
| 5–9 | 1,487 (20.4) | 89 (27.8) |
| ≥ 10 | 1,564 (21.5) | 157 (49.1) |
| Procedures (according to the electric medical records) | ||
| Invasive | 6,696 (91.9) | 316 (98.8) |
| Non-invasive | 589 (8.1) | 4 (1.3) |
| Invasive procedures | ||
| Epidural block | 1,615 (22.1) | 108 (33.8) |
| Arthrocentesis | 301 (4.1) | 34 (10.6) |
| Nerve block | 3,504 (48.1) | 236 (73.8) |
| Block of the peripheral branch of spinal nerve | 5,148 (70.6) | 297 (92.8) |
| Other* | 3,251 (44.6) | 145 (45.3) |
Data are presented as n (%).
* Other: Invasive procedures such as myofascial trigger point injection therapy, blocks of the brain nerve and peripheral branch of the brain nerve, subcutaneous injection, platelet rich plasma, and turbinate cautery, guttering for ingrowing nail, suction drainage or tracheostomy suction, cornea removal, skin electric cauterization.
HCV = hepatitis C virus.
- 1. World Health Organization [Internet]. Hepatitis C. Fact sheet [updated 2018 Jul 18]. Available from: http://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
- 2. Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare [Internet]. 2016 Korea Health Statistics 2017 Available from: https://knhanes.cdc.go.kr/knhanes/sub04/sub04_03.do?classType=7.
- 3. Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare [Internet]. 2017 Infectious Diseases Surveillance Yearbook 2018 Available from: http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=631.
- 4. Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare [Internet]. 2018 Infectious Diseases Surveillance Yearbook 2019 Available from: http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=1873.
- 5. Korean Association for the Study of the Liver [Internet]. Hepatitis C Treatment Guideline 2015;Available from: https://www.kasl.org/bbs/index.html?code=guide&page=1&number=2798&mode=view.
- 6. World Health Organization [Internet]. Global Hepatitis Report 2017. Geneva: 2017. Available from: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
- 7. Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare [Internet]. 2016 Infectious Diseases Epidemiological Investigation Yearbook 2017 Available from: http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=5&pblctDtaSn=23.
- 8. Houri AM, Armstrong GL, Huntin YJ. The Global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS 2004;15(1). 7−16. PMID: 10.1258/095646204322637182.ArticlePubMed
- 9. Center for Disease Control and Prevention [Internet]. Healthcare-Associated Hepatitis B and C Outbreaks (≥ 2 cases) Reported to the Centers for Disease Control and Prevention (CDC) in 2008–2017 Available from: https://www.cdc.gov/hepatitis/outbreaks/pdfs/healthcareinvestigationtable.pdf.
- 10. Kim YS, Ahn YO, Kim DW. A case-control study on the risk factors of hepatitis C virus infection among Koreans. J Korean Med Sci 1996;11(1). 38−43. PMID: 10.3346/jkms.1996.11.1.38. PMID: 8703369. PMID: 3053912.ArticlePubMedPMC
- 11. Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare. Epidemiology and Control of Transfusion-Transmitted Infections 2016.
- 12. Seong MH, Kil H, Kim YS, et al. Clinical and epidemiological features of hepatitis C virus infection in South Korea: A prospective, multicenter cohort study. J Med Virol 2013;85(10). 1724−33. PMID: 10.1002/jmv.23661. PMID: 23813472.ArticlePubMed
- 13. Ryoo SM, Kim WY, Kim W, et al. Transmission of hepatitis C virus by occupational percutaneous injuries in South Korea. J Formos Med Assoc 2012;111(2). 113−7. PMID: 10.1016/j.jfma.2011.05.005. PMID: 22370291.ArticlePubMed
- 14. Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol 2008;48(1). 148−62. PMID: 10.1016/j.jhep.2007.07.033.ArticlePubMed
- 15. Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol 2007;42(7). 513−21. PMID: 10.1007/s00535-007-2064-6. PMID: 17653645.ArticlePubMed
- 16. Shin HR, Kim JY, Kim JI, et al. Hepatitis B and C virus prevalence in a rural area of South Korea: The role of acupuncture. Br J Cancer 2002;87:314−8. PMID: 10.1038/sj.bjc.6600436. PMID: 12177801. PMID: 2364222.ArticlePubMedPMC
- 17. Cho EJ, Jeong SH, Han BH, et al. Hepatitis C virus (HCV) genotypes and the influence of HCV subtype 1b on the progression of chronic hepatitis C in Korea: A single center experience. Clin Mol Hepatol 2012;18(2). 219−24. PMID: 10.3350/cmh.2012.18.2.219. PMID: 22893873. PMID: 3415883.ArticlePubMedPMC
- 18. Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare [Internet]. Standard Guidelines for the Control and Prevention of Healthcare-Associated Infections 2017 Available from: http://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019.
References
Figure & Data
References
Citations



 PubReader
PubReader ePub Link
ePub Link Cite
Cite