Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 7(6); 2016 > Article
-
Original Article
Epidemiology and Inequality in the Incidence and Mortality of Nasopharynx Cancer in Asia - Neda Mahdavifara, Mahshid Ghonchehb, Abdollah Mohammadian-Hafshejanic, Bahman Khosravid, Hamid Salehiniyae,f
-
Osong Public Health and Research Perspectives 2016;7(6):360-372.
DOI: https://doi.org/10.1016/j.phrp.2016.11.002
Published online: November 16, 2016
aHealth Promotion Research Center, Department of Epidemiology and Biostatistics, School of Public Health, Zahedan University of Medical Sciences, Zahedan, Iran
bDepartment of Epidemiology and Biostatistics, School of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran
cDepartment of Social Medicine, School of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
dTehran University of Medical Sciences, Tehran, Iran
eZabol University of Medical Sciences, Zabol, Iran
fDepartment of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- ∗Corresponding author. alesaleh70@yahoo.com
Copyright © 2016 Korea Centers for Disease Control and Prevention. Published by Elsevier Korea LLC.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- One of the most common head and neck cancers is nasopharynx cancer. Knowledge about the incidence and mortality of this disease and its distribution in terms of geographical areas is necessary for further study and better planning. Therefore, this study was conducted with the aim of determining the incidence and mortality rates of nasopharynx cancer and its relationship with the Human Development Index (HDI) in Asia in 2012.
-
Methods
- The aim of this ecologic study was to assess the correlation between age-specific incidence rate (ASIR) and age-specific mortality rate (ASMR) with HDI and its components, which include the following: life expectancy at birth, mean years of schooling, and gross national income per capita. Data about SIR and SMR for every Asian country for 2012 were obtained from the global cancer project. We used the correlation bivariate method for the assessment. Statistical significance was assumed if p < 0.05. All reported p values are two-sided. Statistical analyses were performed using SPSS (Version 15.0, SPSS Inc.).
-
Results
- A total of 68,272 cases (males, 71.02%; females, 28.97%; sex ratio, 2.45) and 40,530 mortalities (males, 71.63%; females, 28.36%; sex ratio, 2.52) were recorded in Asian countries in 2012. The five countries with the highest ASIR of nasopharynx cancer were Malaysia, Singapore, Indonesia, Vietnam, and Brunei, and the five countries with the highest ASMR were Indonesia, Vietnam, Singapore, Malaysia, and Brunei. The correlation between HDI and ASIR was 0.097 (p = 0.520) [0.105 in men (p = 0.488) and 0.119 in women (p = 0.901)]. The correlation between HDI and ASMR was –0.102 (p = 0.502) [–0.072 in men (p = 0.633) and –0.224 in women (p = 0.134)].
-
Conclusion
- Nasopharynx cancer is native to Southeast Asia. The highest incidence and mortality rates are found in Malaysia, Singapore, Indonesia, Vietnam, and Brunei. No significant relation was found between the standardized incidence and mortality rates of nasopharynx cancer and the HDI components. Further studies are recommended in Southeast Asian countries in order to find the etiology of cancer, as well as its diagnosis and treatment.
- Cancer is the leading cause of mortality, with a high financial burden, and one of the major public health concerns at the international level 1, 2. Head and neck malignancies are among the relatively common cancers in humans that affect several anatomic sites of the head and the neck [3]. One of the most common head and neck cancers is nasopharynx cancer 4, 5, which has a very unique distribution pattern. Worldwide, about 86,500 cases of nasopharynx cancer and 50,000 deaths arising from it [6] are reported annually. According to the International Agency for Research on Cancer report in 2008, more than 80% of patients with nasopharynx cancer are in Asia, and only 5% of these cancers are reported in Europe. Specifically, 71% of new nasopharynx cancer cases are recorded in East and Southeast Asia, and 29% are diagnosed in South and Central Asia and North and East Africa [7].
- Nasopharynx cancer is native to Southeast Asia, where the prevalence rate is 15–50 cases per 100,000 people [8]. For the United States and the rest of the world, the incidence of less than 1 case per 100,000 people per year is reported [9]. In addition to geographic diversity, it seems that some ethnic groups are prone to nasopharynx cancer. These groups include the Bidayuh in Borneo, the Nagas in northern India, and the Inuits in the North pole, which have an age-standardized incidence rate of more than 16 per 100,000 people among men each year [10].
- In terms of heterogeneous epidemiological patterns, in addition to nonenvironmental risk factors such as sex, ethnicity, and family history [11], other factors—such as smoking [12], salted fish consumption, especially in childhood 12, 13, 14, 15, 16, 17, 18, nitrosamine in some food items traditionally used in southern China 19, 20, and use of traditional herbal medicines in the Asian population 12, 21, 22, 23, as well as nonfood risk factors such as occupational exposures to formaldehyde, wood dust, smoke, and chemicals 7, 12, 22, 24—may be involved in the pathogenesis of nasopharynx carcinoma [25]. Nasopharynx cancer, in comparison to other head and neck tumors, has different epidemiological, staging, and treatment characteristics. Most patients are diagnosed when they are in advanced stages 26, 27.
- The Human Development Index (HDI) is a useful tool to compare the incidence and mortality rates of cancer at the global level 28, 29, 30 and is one of the indicators to check the status of illnesses and deaths between countries. In fact, this index has been observed to be related with the incidence and mortality rates of many diseases; it is considered a good index to obtain information regarding the status of a specific disease in different countries. The HDI is composed of three basic dimensions: life expectancy at birth, adult literacy rate, and gross domestic product (GDP) per capita. The relationship between HDI and some cancers is studied, and investigating this relationship can lead to a more accurate understanding of cancer and its risk factors distribution [31], and it is also suggested to be used for other cancers. Although nutritional and communicable diseases are common causes of death in countries with a low HDI, it is anticipated that by 2030, one common cause of death in these countries will be noncommunicable diseases such as cancer [32]. Because awareness about nasopharynx cancer incidence and mortality can be useful for health programs and research activities, and considering the possible role of the HDI, this study was conducted with the aim of evaluating the incidence and mortality of nasopharynx carcinoma and its relationship with the development index and its components in Asia in 2012.
Introduction
- We conducted an ecologic study in Asia to assess the correlation between age-specific incidence and mortality rate (ASR) with HDI and its components, which include the following: life expectancy at birth, mean years of schooling, and gross national income (GNI) per capita. The ASR data of all Asian countries for the year 2012 were obtained from the global cancer project (available at http://globocan.iarc.fr/Default.aspx) [33], and the HDI data was based on the Human Development Report 2013 [34], which contains information about HDI and its components for every country in the world in 2012.
- 2.1 Method of estimating ASRs in global cancer project by the International Agency for Research on Cancer 2.1.1
- The methods of estimation are country-specific, and the quality of the estimation depends on the quality and amount of information available for each country. In theory, there are as many methods as countries, and because of the variety and the complexity of these methods, an overall quality score for the incidence and mortality estimates combined is almost impossible to establish. However, an alphanumeric scoring system that independently describes the availability of incidence and mortality data has been established at the country level. The combined score is presented together with the estimates for each country with the aim of providing a broad indication of the robustness of the estimation.
- The methods used to estimate the sex- and age-specific incidence rates of cancer for a specific country fall into one of the following broad categories (by order of priority):
1. Rates projected to 2012 (38 countries).
2. Most recent rates applied to 2012 population (20 countries).
3. Estimated from national mortality by modeling, using incidence mortality ratios derived from recorded data in country-specific cancer registries (13 countries).
4. Estimated from national mortality estimates by modeling, using incidence mortality ratios derived from recorded data in local cancer registries in neighboring countries (9 European countries).
5. Estimated from national mortality estimates using modeled survival (32 countries)
6. Estimated as the weighted average of the local rates (16 countries)
7. One cancer registry covering part of a country is used as representative of the country profile (11 countries).
8. Age/sex-specific rates for “all cancers” were partitioned using data on relative frequency of different cancers (by age and sex) (12 countries).
9. The rates are those of neighboring countries or registries in the same area (33 countries) 33, 35.
2.1.2 - Depending on the degree of detail and accuracy of the national mortality data, six methods were used in the following order of priority:
1. Rates projected to 2012 (69 countries).
2. Most recent rates applied to 2012 population (26 countries).
3. Estimated as the weighted average of regional rates (1 country).
4. Estimated from national incidence estimates by modeling, using country-specific survival (2 countries).
5. Estimated from national incidence estimates using modeled survival (83 countries).
6. The rates are those of neighboring countries or registries in the same area (3 countries) 33, 35.
- 2.2 Human Development Index
- HDI is a composite measure of indicators along three dimensions: life expectancy, educational attainment, and command over the resources needed for a decent living. All groups and regions have seen notable improvement in all HDI components, with faster progress in low and medium HDI countries. On this basis, the world is becoming less unequal. Nevertheless, national averages hide large variations in human experience. Wide disparities remain within countries of both the North and the South, and income inequality within and between many countries has been rising [34].
- 2.3 Statistical analysis
- In this study, we used the correlation bivariate method to assess the correlation between ASR and HDI and its components (life expectancy at birth, mean years of schooling, and GNI per capita). Statistical significance was assumed if p < 0.05. All reported p values are two-sided. Statistical analyses were performed using SPSS (Version 15.0: SPSS Inc., Chicago).
Materials and methods
2.1.1 Age-specific incidence rate estimate
2.1.2 Age-specific mortality rate estimate
- In general, a total of 68,272 nasopharynx cancer cases were recorded in Asian countries in 2012, of which 48,492 cases (2/71%) were diagnosed in men and 19,780 cases (97/28%) were diagnosed in women. These figures indicate that the sex (male/female) ratio is 45:2. Five countries with the highest number of new cases of nasopharynx cancer are as follows: (1) China with 33,198 cases; (2) Indonesia, 13,084 cases; (3) Vietnam, 4,931 cases; (4) India, 3,947 cases; (5) Malaysia, 2,030 cases; these five countries alone accounted for a total of 57,190 cases (76/83%) of all cases in Asia. In Asia, the five countries with the highest standardized incidence of nasopharynx cancer are as follows: (1) Malaysia, with a standardized rate of 7.2 per 100,000 people; (2) Singapore, 6.4 per 100,000 people; (3) Indonesia, 5.6 per 100,000 people; (4) Vietnam, 5.4 per 100,000 people; (5) Brunei, 5 per 100,000 people. Conversely, the five countries with the lowest standardized incidence of nasopharynx cancer are as follows: (1) Maldives, with a rate of 0 per 100,000 people; (2) Tajikistan, 0.1 per 100,000 people; (3) Japan, 0.2 per 100,000 people; (4) Bahrain, 0.2 per 100,000 people; (5) Uzbekistan, 0.3 per 100,000 people. The number and rate of standardized and crude incidence of this cancer in Asian countries according to sex are shown in Table 1. Countries are arranged based on standardized rates, from highest to lowest, in Table 1, so that countries with the highest and lowest standardized incidence rates in each sex are shown (Table 1, Figure 1, Figure 2).
- Of the 40,530 people who died of nasopharynx cancer in Asia in 2012, 29,032 (63/71%) were men and 11,498 (36/28%) were women, which translates to a male/female mortality ratio of 52:2. The highest number of deaths occurred in China with 20,404 cases, followed by Indonesia with 7,391 cases, Vietnam with 2,885 cases, India with 2,836 cases, and Thailand with 1,114 cases. This brings the total of deaths to 34,630 (44/85%) in just these five countries.
- The five countries with the highest standardized mortality rate of nasopharynx cancer are as follows: (1) Indonesia, with a standardized rate of 3.3 per 100,000 people; (2) Vietnam, 3.3 per 1000,000 people; (3) Singapore, 2.8 per 100,000 people; (4) Malaysia, 2.5 per 100,000 people; (5) Brunei, 2.1 per 100,000 people. Conversely, the five countries with the lowest standardized mortality rate of nasopharynx cancer are as follows: Maldives and Tajikistan, with a rate of 0 per 100,000 people; followed by Bahrain, Kuwait, and Israel with a rate of 0.1 per 100,000 people. The number and rate of standardized and crude mortality of this cancer in Asian countries by sex are shown in Table 2. Countries are arranged based on standardized rates, from highest to lowest, in Table 2, so that countries with the highest and lowest standardized rates in each sex are shown (Table 2 and Figure 2, Figure 3).
- In Table 3, values of HDI and its components for all Asian countries, arranged according to HDI, are shown. Thus, Asian countries were classified in terms of HDI as follows: three countries in very high category, four countries in top category, 35 countries in the medium category, three countries in low category, and a country in uncertain category.
- 3.1 Checking the relationship between standardized incidence rate and HDI
- A correlation of 0.097 was obtained between the standardized incidence rate of nasopharynx cancer and HDI; however, this relation was not statistically significant (p = 0.520). As for the correlation between components of the HDI and the standardized rate, we obtained the following results: a correlation of 0.174 between the standardized incidence rate and life expectancy at birth (p = 0.247), a negative correlation of 0.057 with the average years of schooling (p = 0.705), and a correlation of 0.134 with the level of income per person of the population (p = 0.375; Figure 4).
- In men, a correlation of 0.105 was observed between the standardized incidence rate of nasopharynx cancer and the HDI; however, this relation was not statistically significant (p = 0.488).
- A correlation was also seen between the components of the HDI and the standardized incidence. Specifically, we obtained a correlation of 0.174 between the standardized incidence rate and life expectancy at birth (p = 0.253), a negative correlation of 0.031 with the average years of schooling (p = 0.836), and a correlation of 0.125 with the level of income per person of the population (p = 0.407).
- In women, a correlation of 0.019 was found between the standardized incidence rate of nasopharynx cancer and the HDI, but this relation was not statistically significant (p = 0.901). A correlation between components of the HDI and the standardized incidence rate was also found. Specifically, we noted a correlation of 0.134 between the standardized incidence rate and life expectancy at birth (p = 0.376), a negative correlation of 0.142 with the average years of schooling (p = 0.346), and a correlation of 0.059 with the level of income per person of the population (p = 0.696).
- 3.2 Checking the relationship between standardized mortality rate and HDI
- A negative correlation of 0.102 was found between the standardized mortality rate of nasopharynx cancer and HDI; however, this relation was not statistically significant (p = 0.502). A correlation was also found between components of the HDI and the standardized rate. Specifically, we noted a correlation of 0.034 between the standardized incidence rate and life expectancy at birth (p = 0.824), a negative correlation of 0.238 with the average years of schooling (p = 0.112), and a correlation of 0.001 with the level of income per person of the population (p = 0.994; Figure 5).
- In men, we observed a correlation of 0.072 between the standardized mortality rate of nasopharynx cancer and the HDI, but this relation was not statistically significant (p = 0.633). Furthermore, a correlation between components of the HDI and standardized mortality rate was also found. Specifically, we noted a correlation of 0.040 between the standardized incidence rate and life expectancy at birth (p = 0.792), a negative correlation of 0.188 with the average years of schooling (p = 0.211), and a correlation of 0.014 with the level of income per person of the population (p = 0.924).
- In women, a negative correlation of 0.224 was found between the standardized mortality rate of nasopharynx cancer and the HDI, but this relation was not statistically significant (p = 0.134). Also, a negative correlation was noted between components of the HDI and the standardized rate. Specifically, we observed a negative correlation of 0.044 between the standardized incidence rate and life expectancy at birth (p = 0.773), a negative correlation of 0.345 with the average years of schooling (p = 0.019), and a negative correlation of 0.014 with the level of income per person of the population (p = 0.350).
Results
- Overall, 68,272 new cases and 40,530 deaths attributed to nasopharynx cancer were recorded in Asian countries in 2012; the sex (male/female) ratio of developing the disease is 2.45, and the sex ratio of nasopharynx cancer mortality is 2.52. The prevalence of nasopharynx cancer in developing countries is higher, and this is attributed to the higher exposure of inhabitants to a variety of risk factors and lower budget allocations for health. For these people, diagnosis is often made when the disease is already in advanced stages, and, with the lack of access to treatment, the metastasis of nasopharynx cancer can be observed more often in these areas 36, 37.
- In this study, no significant relationship was found between the standardized incidence and mortality rate of nasopharynx cancer and the HDI. Moreover, no significant positive relationship was observed between standardized incidence and mortality rate of nasopharynx cancer with proper income level (GDP) and level of education or knowledge (mean years of schooling). In other studies, the increase in life expectancy leads to an increase in global cancer burden and future changes in population growth and aging, indicating that new cases of nasopharynx cancer and mortality in elderly people will increase and that this increase will be noticeable in countries with low HDI compared to countries with high HDI (76% vs. 25%) 26, 28, 38, 39. Furthermore, according to other studies, people who have higher education typically have more healthy habits and behaviors than those with a low education level, especially in terms of cancer incidence and mortality 40, 41. In studies focusing on nasopharynx cancer, the relationship between nasopharynx cancer with socioeconomic status, lifestyle, and geographical positions is known [12]. Thus, in Asian countries, there is an increased incidence of nasopharynx cancer in lower economic classes, especially among men [12]. Also, no difference was found between the deaths of people living in rural areas with low socioeconomic status and the people who live in urban areas 42, 43.
- In this study, Malaysia, Singapore, Indonesia, Vietnam, and Brunei are the five countries with the highest incidence rate. It is noteworthy that Singapore and Brunei are classified as very high HDI countries, Malaysia is considered a country with a high HDI, and Indonesia and Vietnam are countries with medium HDI. According to previous studies in Singapore, nasopharynx cancer is the eighth most common cancer in men, with age-standardized incidence of 9.5 per 100,000 per year [44]. In Indonesia, a relatively high incidence is reported—at least 5.7 among men and 1.9 among women per 100,000 people—compared with the global incidence average of 1.9 among men and 0.8 among women for every 100,000 individuals [45]. It should be noted, however, that the actual incidence of nasopharynx cancer in Indonesia is not known owing to incomplete registration [11].
- Regardless of ethnicity, genetics plays an important part in the pathogenesis of nasopharynx cancer. The incidence of nasopharynx cancer is 20–50 times higher in South China compared with Western countries. It is notable that second and third generations of Chinese people who immigrated to the United States (a low prevalence area) are still at risk of high nasopharynx cancer despite cultural assimilation compared with the resident population [46]. In the present study, Indonesia, Vietnam, Singapore, Malaysia, and Brunei were the five countries with the highest death rates from this cancer. Other studies have shown that one of the main causes of death in Indonesia is nasopharynx cancer, and in early detection, 80% of the patients are already at an advanced stage of the disease. In Indonesia, primary healthcare is generally handled by health centers named Puskesmas. Lack of knowledge among the general practitioners working in these centers regarding the various aspects of nasopharynx cancer, may lead to a delay in diagnosis [11]. Thus, only about 14% of patients who have metastasis at the initial diagnosis and 29% of patients without metastasis show response to therapy, about 70–95% response to therapy is good, so in general response to the therapy is poor. [36]. In Singapore, older patients diagnosed with stage 2 or stage 3 are at higher risk of recurrence and lower overall survival [47]. Eighty percent of patients are now at stage 3 or 4 of the disease, which leads to lower survival and death [11]. In the past two decades, the treatment of nasopharynx cancer has been considerably enhanced by the use of chemotherapy and radiotherapy at the same time. However, the overall incidence of patients with metastasis remains at 25–34%, and survival of these patients is low 48, 49.
Discussion
- Nasopharynx cancer is the native cancer of Southeast Asia. So that the highest incidence and mortality are related to Southeast Asian countries including Malaysia, Singapore, Indonesia, Vietnam and Brunei. It was not seen a significant relation between the standardized incidence and mortality rate of nasopharynx cancer and the Human Development Index components. Further studies are recommended in Southeast Asian countries in order to find the etiology of cancer, diagnosis and treatment.
- 5.1 Limitations of the study
- This was an ecological study. The ecological fallacy will occur if results are inferred and concluded at the individual level.
Conclusion
- The authors declare that they have no conflicts of interest for this work.
Conflicts of interest
-
Acknowledgements
- We appreciate the cooperation of all employees involved in data collection in the GLOBOCAN project and World Bank.
Acknowledgments
- 1. Keyghobadi N., Rafiemanesh H., Mohammadian-Hafshejani A.. Epidemiology and trend of cancers in the province of Kerman: southeast of Iran. Asian Pac J Cancer Prev 16(4). 2015 Apr;1409−1413. PMID: 25743807.ArticlePubMed
- 2. Razi S., Rafiemanesh H., Ghoncheh M.. Changing trends of types of skin cancer in Iran. Asian Pac J Cancer Prev 16(12). 2015 Dec;4955−4958. PMID: 26163621.ArticlePubMed
- 3. Edited by Devita V.T., Hellman S., Rosenberg S.A.: Principles and practice of oncology. 2001. Lippincott, Williams &Wilkims; Philadelphia (PA): pp 1880−1904.
- 4. Tsao S.W.L.K., Huang D.P.. Nasopharyngeal carcinoma. Edited by Tselis A.C., Jenson H.: Epstein–Barr virus. 2006. Taylor & Francis; New York (NY): pp 273−295.
- 5. Raab-Traub N.. Epstein–Barr virus in the pathogenesis of NPC. Edited by Robertson E.S.: Epstein–Barr virus. 2005. Caister Academic Press; Wymondham Norfolk (UK): pp 71−92.
- 6. Parkin D.M., Bray F., Ferlay J.. Global cancer statistics, 2002. CA Cancer J Clin 55(2). 2005 Mar-Apr;74−108. PMID: 15761078.ArticlePubMed
- 7. Chang E.T., Adami H.-O.. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomark Prev 15(10). 2006 Oct;1765−1777.Article
- 8. Rubin P., Bakemeier R., Kiackov S.. Clinical oncology. 6th ed.1983. National Cancer Institute; USA: pp 2−10.
- 9. Wang Y., Zhang Y., Ma S.. Racial differences in nasopharyngeal carcinoma in the United States. Cancer Epidemiol 37(6). 2013 Dec;793−802. PMID: 24035238.ArticlePubMed
- 10. Wee J., Ha T.C., Loong S., Qian C.. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer 29(5). 2010 May;517−526. PMID: 20426903.ArticlePubMed
- 11. Fles R., Wildeman M.A., Sulistiono B.. Knowledge of general practitioners about nasopharyngeal cancer at the Puskesmas in Yogyakarta, Indonesia. BMC Med Educ 10(1). 2010 Nov;1PMID: 20074350.ArticlePubMed
- 12. Mimi C.Y., Yuan J.-M.. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 12(6). Dec 2002;421−426. PMID: 12450728.ArticlePubMed
- 13. Mimi C.Y., Ho J.H., Lai S.-H.. Cantonese-style salted fish as a cause of nasopharyngeal carcinoma: report of a case-control study in Hong Kong. Cancer Res 46(2). 1986 Feb;956−961. PMID: 3940655.PubMed
- 14. Yu M., Mo C., Chong W.. Preserved foods and nasopharyngeal carcinoma: a case-control study in Guangxi, China. Cancer Res 48(7). 1988 Apr;1954−1959. PMID: 3349469.PubMed
- 15. Yu M.C., Huang T.B., Henderson B.E.. Diet and nasopharyngeal carcinoma: a case-control study in Guangzhou, China. Int J cancer 43(6). 1989 Jun;1077−1082. PMID: 2732001.ArticlePubMed
- 16. Ning J.-P., Mimi C.Y., Wang Q.-S.. Consumption of salted fish and other risk factors for nasopharyngeal carcinoma (NPC) in Tianjin, a low-risk region for NPC in the People's Republic of China. J Natl Cancer Inst 82(4). 1990 Feb;291−296. PMID: 2299678.ArticlePubMed
- 17. Aiyar A., Tyree C., Sugden B.. The plasmid replicon of EBV consists of multiple cis-acting elements that facilitate DNA synthesis by the cell and a viral maintenance element. EMBO J 17(21). 1998 Nov;6394−6403. PMID: 9799247.ArticlePubMed
- 18. Ho J.. Genetic and environmental factors in nasopharyngeal carcinoma. Recent advances in human tumor virology and immunology. 1971. University of Tokyo Press; Tokyo (Japan): pp 275−295.
- 19. Zou X.N., Lu S.H., Liu B.. Volatile N-nitrosamines and their precursors in chinese salted fish—a possible etological factor for NPC in China. Int J Cancer 59(2). 1994 Oct;155−158. PMID: 7927911.ArticlePubMed
- 20. Huang D., Ho J., Webb K.. Volatile nitrosamines in salt-preserved fish before and after cooking. Food Cosmet Toxicol 19:1981 Apr;167−171. PMID: 7286866.ArticlePubMed
- 21. Zheng Y., Tuppin P., Hubert A.. Environmental and dietary risk factors for nasopharyngeal carcinoma: a case-control study in Zangwu County, Guangxi, China. Br J Cancer 69(3). 1994 Mar;508−514. PMID: 8123482.ArticlePubMed
- 22. West S., Hildesheim A., Dosemeci M.. Non-viral risk factors for nasopharyngeal carcinoma in the philippines: results from a case-control study. Int J Cancer 55(5). 1993 Nov;722−727. PMID: 7503957.ArticlePubMed
- 23. Hildesheim A., West S., DeVeyra E.. Herbal medicine use, Epstein–Barr virus, and risk of nasopharyngeal carcinoma. Cancer Res 52(11). 1992 Jun;3048−3051. PMID: 1317256.PubMed
- 24. Hildesheim A., Dosemeci M., Chan C.-C.. Occupational exposure to wood, formaldehyde, and solvents and risk of nasopharyngeal carcinoma. Cancer Epidemiol Biomark Prev 10(11). 2001 Nov;1145−1153.
- 25. Chua M.L., Wee J.T., Hui E.P.. Nasopharyngeal carcinoma. Lancet 387(10022). 2016 Mar;1012−1024. PMID: 26321262.ArticlePubMed
- 26. Chan A.T., TP , Johnson P.J.. Nasopharyngeal carcinoma. Ann Oncol 13:2002 Jul;1007−1015. PMID: 12176778.ArticlePubMed
- 27. Agulnik M., Siu L.. State-of-the-art management of nasopharyngeal carcinoma: current and future directions. Br J Cancer 92(5). 2005 Mar;799−806. PMID: 15756250.ArticlePubMed
- 28. Bray F., Jemal A., Grey N.. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 13(8). 2012 Aug;790−801. PMID: 22658655.ArticlePubMed
- 29. Ghoncheh M., Mohammadian-Hafshejani A., Salehiniya H.. Incidence and mortality of breast cancer and their relationship to development in Asia. Asian Pac J Cancer Prev 16(14). 2015 Dec;6081−6087. PMID: 26320499.ArticlePubMed
- 30. Ghoncheh M., Mirzaei M., Salehiniya H.. Incidence and mortality of breast cancer and their relationship with the human development index (HDI) in the world in 2012. Asian Pac J Cancer Prev 16(18). 2015 Dec;8439−8443. PMID: 26745098.ArticlePubMed
- 31. Pakzad R., Mohammadian-Hafshejani A., Ghoncheh M., Pakzad I., Salehiniya H.. The incidence and mortality of lung cancer and their relationship to development in Asia. Translational lung cancer research 4(6). 2015;763−774. PMID: 26798586.PubMedPMC
- 32. Wagner K.H., Brath H.. A global view on the development of non communicable diseases. Prev Med 54:2012 May;S38−S41. PMID: 22178469.ArticlePubMed
- 33. Ferlay J., Soerjomataram I., Ervik M.. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC Cancer Base No. 11 [Internet]. 2013. International Agency for Research on Cancer; Lyon (France): [cited 2016 Feb 2]. Available from:. http://globocan.iarc.fr.
- 34. Malik K.. Human development report 2013. The rise of the south: Human progress in a diverse world (March 15, 2013) UNDP–HDRO Human Development Reports. 2013.
- 35. Ferlay J., Soerjomataram I., Dikshit R.. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5). 2015 Mar;E359−E386. PMID: 25220842.ArticlePubMed
- 36. Adham M., Stoker S.D., Wildeman M.A.. Current status of cancer care for young patients with nasopharyngeal carcinoma in Jakarta, Indonesia. PloS One 9(7). 2014 July;e102353PMID: 25019625.ArticlePubMed
- 37. Wildeman M.A., Fles R., Herdini C.. Primary treatment results of nasopharyngeal carcinoma (NPC) in Yogyakarta, Indonesia. PloS One 8(5). 2013 May;e63706PMID: 23675501.ArticlePubMed
- 38. Giovino G.A., Mirza S.A., Samet J.M.. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 380(9842). 2012 Aug;668−679. PMID: 22901888.ArticlePubMed
- 39. Mirzaei M., Hosseini S.A., Ghoncheh M.. Epidemiology and trend of head and neck cancers in Iran. Global J Health Sci 8(1). 2016 May;189−193.Article
- 40. Stelmach W., Kaczmarczyk-Chalas K., Bielecki W.. The impact of income, education and health on lifestyle in a large urban population of Poland (Cindi programme). Int J Occup Med Environ Health 17(3). 2004 Feb;393−401. PMID: 15683160.PubMed
- 41. Shi L.. Sociodemographic characteristics and individual health behaviors. Southern Med J 91(10). 1998 Oct;933−941. PMID: 9786288.ArticlePubMed
- 42. Mimi C.Y., Ho J.H., Ross R.K.. Nasopharyngeal carcinoma in Chinese—salted fish or inhaled smoke? Prev Med 10(1). 1981 Jan;15−24. PMID: 7232343.ArticlePubMed
- 43. Armstrong R., Kutty M.K., Dharmalingam S.. Incidence of nasopharyngeal carcinoma in Malaysia, 1968–1977. Br J Cancer 40(4). 1979 Oct;557−567. PMID: 497106.ArticlePubMed
- 44. Teo M.C., Soo K.C.. Cancer trends and incidences in Singapore. Jpn J Clin Oncol 43(3). 2013 Mar;219−224. PMID: 23303840.ArticlePubMed
- 45. Rafiemanesh H., Mehtarpoor M., Mohammadian-Hafshejani A.. Cancer epidemiology and trends in Sistan and Baluchestan province. Iran. Med J Islamic Rep Iran 29:2015 Aug;254.
- 46. Buell P.. The effect of migration on the risk of nasopharyngeal cancer among Chinese. Cancer Res 34(5). 1974;1189−1191. PMID: 4842361.PubMed
- 47. Mak H.W., Lee S.H., Chee J.. Clinical outcome among nasopharyngeal cancer patients in a multi-ethnic society in Singapore. PloS One 10(5). 2015;e0126108PMID: 25965270.ArticlePubMedPMC
- 48. Chen M.-Y., Jiang R., Guo L.. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer 32(11). 2013;604−613. PMID: 24206918.ArticlePubMedPMC
- 49. Lee A.W., Lin J.C., Ng W.T.. Current management of nasopharyngeal cancer. Semin Radiat Oncol 22(3). 2012 July;233−244. PMID: 22687948.ArticlePubMed
References
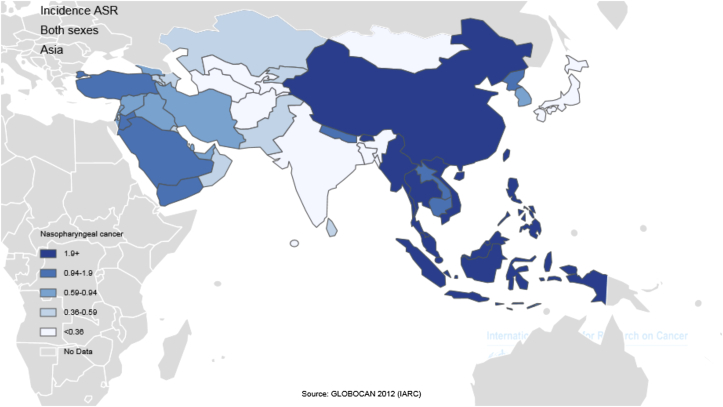
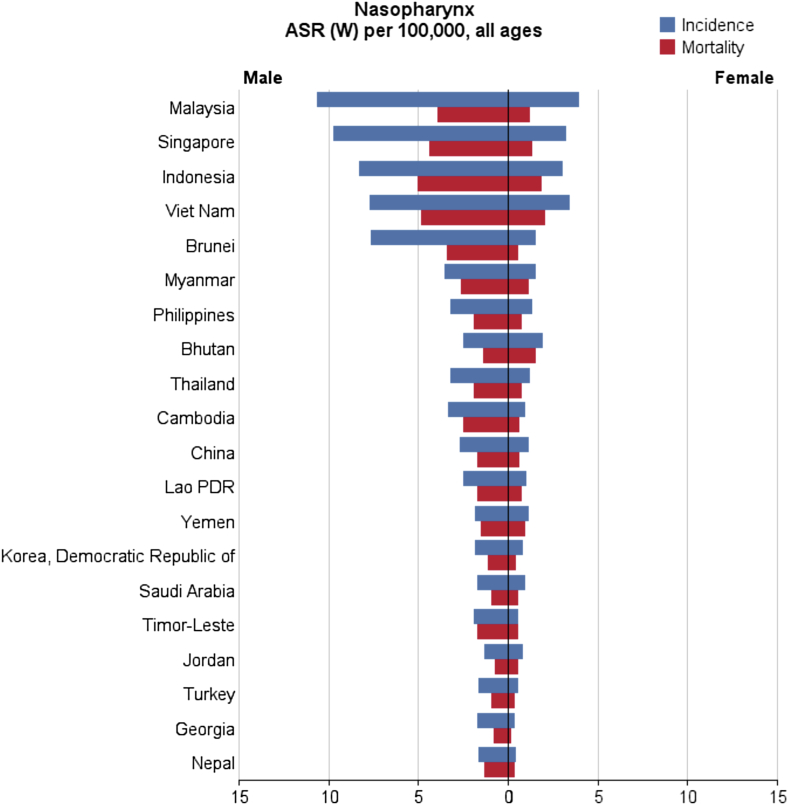
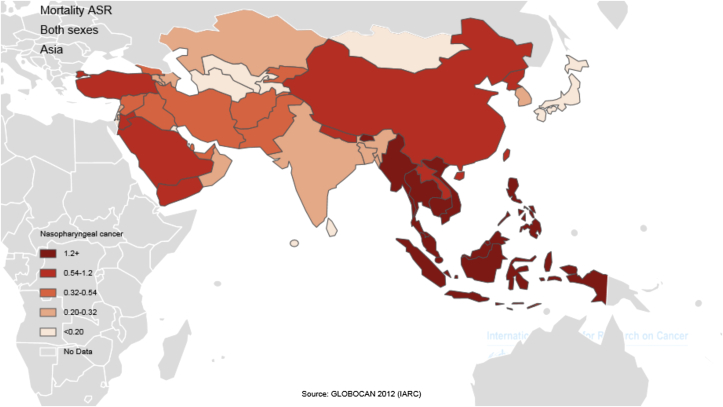
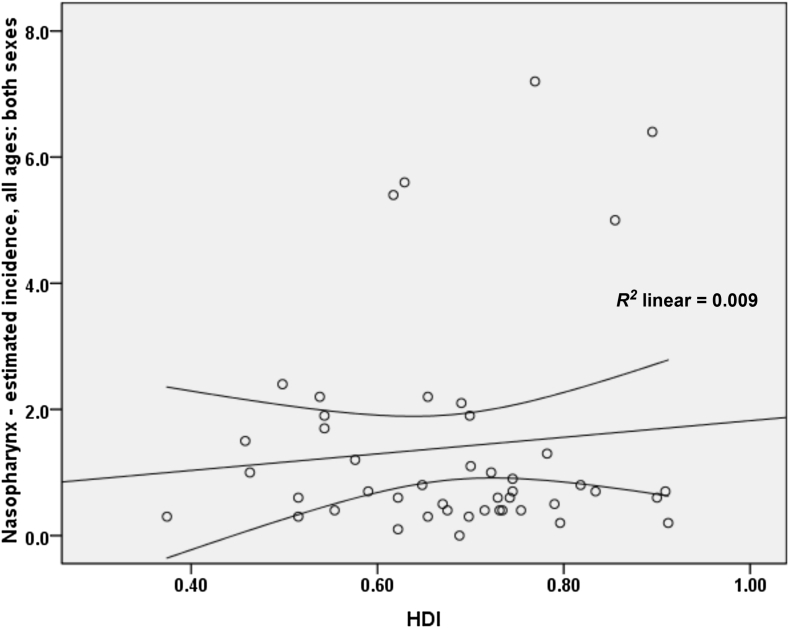

Figure & Data
References
Citations

- Regulatory role of miRNAs in nasopharyngeal cancer involving PTEN/PI3K/AKT, TGFβ/SMAD, RAS/MAPK, Wnt/β‐catenin and pRB‐E2F signaling pathways: A review
Rabiatul Basria S. M. N. Mydin, Adam Azlan, Simon I. Okekpa, Nigel J. Gooderham
Cell Biochemistry and Function.2024;[Epub] CrossRef - Rapid and sensitive detection of Epstein-Barr virus antibodies in nasopharyngeal carcinoma by chemiluminescence strips based on iron-porphyrin single atom nanozyme
Daji Wang, Jie Wang, Dan Liu, Jiuyang He, Meiying Wang, Haibing Huang, Guohui Nie, Hui Ding, Xiyun Yan
Nano Research.2024; 17(3): 1827. CrossRef - The association between chronic sinonasal inflammation and nasopharyngeal carcinoma - A systematic review and meta-analysis
Yuxing Wang, Kylynn Kathleen Koh, Elizabeth Chua, Kimberley Liqin Kiong, Yu Heng Kwan, Tze Choong Charn
American Journal of Otolaryngology.2024; 45(2): 104206. CrossRef - Correlation between socioeconomic indices and epidemiological indices of thyroid cancer from 1990 to 2019 year: a global ecologic study
Zahra Maleki, Jafar Hassanzadeh, Haleh Ghaem
BMC Cancer.2024;[Epub] CrossRef - Study of Three Potential Diagnostic Biomarkers in Nasopharyngeal Carcinoma Samples from Guilan, North of Iran
Saghi Jani Kargar Moghaddam, Amaneh Mohammadi Roushandeh, Mehryar Habibi Roudkenar, Shadman Nemati, Nima Najafi-Ghalehlou, Toofan Pakzad, Masoud Hamidi
International Archives of Otorhinolaryngology.2023; 27(03): e461. CrossRef - Oncogenic Viruses-Encoded microRNAs and Their Role in the Progression of Cancer: Emerging Targets for Antiviral and Anticancer Therapies
Mahmoud Kandeel
Pharmaceuticals.2023; 16(4): 485. CrossRef - Nasopharyngeal Carcinoma Subtype Discovery via Immune Cell Scores from Tumor Microenvironment
Yanbo Sun, Yun Liu, Hanqi Chu, Zhen-Jian Zhuo
Journal of Immunology Research.2023; 2023: 1. CrossRef - The application of Aptamer in biomarker discovery
Yongshu Li, Winnie Wailing TAM, Yuanyuan Yu, Zhenjian Zhuo, Zhichao Xue, Chiman Tsang, Xiaoting Qiao, Xiaokang Wang, Weijing Wang, Yongyi Li, Yanyang Tu, Yunhua Gao
Biomarker Research.2023;[Epub] CrossRef - Blood-based DNA methylation in advanced Nasopharyngeal Carcinoma exhibited distinct CpG methylation signature
Koustav Chatterjee, Sudipa Mal, Monalisha Ghosh, Nabanita Roy Chattopadhyay, Sankar Deb Roy, Koushik Chakraborty, Syamantak Mukherjee, Moatoshi Aier, Tathagata Choudhuri
Scientific Reports.2023;[Epub] CrossRef - Circular RNA circ_0008450 regulates the proliferation, migration, invasion, apoptosis and chemosensitivity of CDDP-resistant nasopharyngeal carcinoma cells by the miR-338-3p/SMAD5 axis
Lin Liu, Bin Lu, Yan Li
Anti-Cancer Drugs.2022; 33(1): e260. CrossRef - Hypermethylation of the RASSF1A gene promoter as the tumor DNA marker for nasopharyngeal carcinoma
Thuan Duc Lao, Hue Hong Thieu, Dung Huu Nguyen, Thuy Ai Huyen Le
The International Journal of Biological Markers.2022; 37(1): 31. CrossRef - miR-135b-5p Targets SIRT1 to Inhibit Deacetylation of c-JUN and Increase MMP7 Expression to Promote Migration and Invasion of Nasopharyngeal Carcinoma Cells
Yali Cheng
Molecular Biotechnology.2022; 64(6): 693. CrossRef - Trends in the Incidence of Nasopharyngeal Cancer in Saudi Arabia Across One Decade (2007 to 2016)
Abdualrahman F Kabli, Khalil F Miyajan, Abdulmohsen S Alqurashi, Ammar K Mandili, Revan M Mujahed, Bayan F Hafiz, Roaa M Mandora, Ameen Z Herabi
Cureus.2022;[Epub] CrossRef - Causes of Death in Long-Term Nasopharyngeal Carcinoma Survivors
Shi-Ping Yang, Ming-Yue Rao, Qing-Shuang Chen, Ping Zhou, Chen-Lu Lian, San-Gang Wu
Frontiers in Public Health.2022;[Epub] CrossRef - Epstein-Barr Virus (EBV) Is Mostly Latent and Clonal in Angioimmunoblastic T Cell Lymphoma (AITL)
Racha Bahri, François Boyer, Mohamad Adnan Halabi, Alain Chaunavel, Jean Feuillard, Arnaud Jaccard, Sylvie Ranger-Rogez
Cancers.2022; 14(12): 2899. CrossRef - Ferroptosis-related gene ATG5 is a novel prognostic biomarker in nasopharyngeal carcinoma and head and neck squamous cell carcinoma
Ming Shi, Jiangnan Du, Jingjing Shi, Yunchuanxiang Huang, Yan Zhao, Lan Ma
Frontiers in Bioengineering and Biotechnology.2022;[Epub] CrossRef - Platelet to Lymphocytes Ratio to Predict Nasopharyngeal Carcinoma Progressivity
Goesti Yudistira, Yussy Afriani Dewi, Melati Sudiro
Open Access Macedonian Journal of Medical Sciences.2022; 10(B): 2189. CrossRef - Skin sparing in intensity-modulated radiation therapy of nasopharyngeal carcinoma
MisbaHamid Baba, BenoyK Singh, Shaq ulQamar Wani
Journal of Medical Physics.2022; 47(3): 243. CrossRef - Assessment of Response to Chemoradiation and Radiation Therapy in Patients with Nasopharyngeal Carcinoma
Sebastian Ario Susanto, Yussy Afriani Dewi, Raden Ayu Hardianti Saputri
Open Access Macedonian Journal of Medical Sciences.2022; 10(B): 2307. CrossRef - Genetic variants in NKG2D axis and susceptibility to Epstein–Barr virus-induced nasopharyngeal carcinoma
Nguyen Hoang Viet, Nguyen Quang Trung, Le Thanh Dong, Ly Quoc Trung, J. Luis Espinoza
Journal of Cancer Research and Clinical Oncology.2021; 147(3): 713. CrossRef - Corticosteroid Therapy in Optic Neuropathy Secondary to Nasopharyngeal Carcinoma
Zulaikha Wahab, Evelyn Tai, Wan-Hazabbah Wan Hitam, Khairy Shamel Sonny Teo
Cureus.2021;[Epub] CrossRef - The Effect of Hispidulin, a Flavonoid from Salvia plebeia, on Human Nasopharyngeal Carcinoma CNE-2Z Cell Proliferation, Migration, Invasion, and Apoptosis
Yiqun Dai, Xiaolong Sun, Bohan Li, Hui Ma, Pingping Wu, Yingping Zhang, Meilin Zhu, Hong-Mei Li, Minjian Qin, Cheng-Zhu Wu
Molecules.2021; 26(6): 1604. CrossRef - δ-Tocotrienol induces apoptosis and inhibits proliferation of nasopharyngeal carcinoma cells
Junjun Shen, Tao Yang, Yiping Tang, Tianyi Guo, Ting Guo, Tao Hu, Feijun Luo, Qinlu Lin
Food & Function.2021; 12(14): 6374. CrossRef - WNT8B as an Independent Prognostic Marker for Nasopharyngeal Carcinoma
Chawalit Ngernsombat, Pongphol Prattapong, Noppadol Larbcharoensub, Krittika Khotthong, Tavan Janvilisri
Current Oncology.2021; 28(4): 2529. CrossRef - Nasopharyngeal Carcinoma and Its Association with Epstein-Barr Virus
Harish N. Vasudevan, Sue S. Yom
Hematology/Oncology Clinics of North America.2021; 35(5): 963. CrossRef - Association between stage and histopathological type of nasopharyngeal cancer on occurrence of postirradiation otitis media with effusion
Lina Lasminingrum, Shinta Fitri Boesoeri, Sally Mahdiani, Eveline Sabrina Ranti
International Journal of Surgery Open.2021; 36: 100376. CrossRef - Current Status and Future Perspectives about Molecular Biomarkers of Nasopharyngeal Carcinoma
Pui Yan Siak, Alan Soo-Beng Khoo, Chee Onn Leong, Boon-Peng Hoh, Shiau-Chuen Cheah
Cancers.2021; 13(14): 3490. CrossRef - Carcinomatous‑like mastitis due to axillary lymphadenopathy in a case of nasopharyngeal carcinoma: A case report
Cristina Oprean, Nusa Segarceanu, Alexandra Stan, Cristian Suciu, Daciana Grujic, Ioana Rivis, Alis Liliana Dema, Ana Bredicean
Experimental and Therapeutic Medicine.2021;[Epub] CrossRef - Dosimetric Comparison of Helical Tomotherapy, Volume-Modulated Arc Therapy, and Fixed-Field Intensity-Modulated Radiation Therapy in Locally Advanced Nasopharyngeal Carcinoma
Shan Lu, Huiqi Fan, Xueyuan Hu, Xin Li, Yingying Kuang, Deyang Yu, Shanshan Yang
Frontiers in Oncology.2021;[Epub] CrossRef - The dosimetric comparison between tomotherapy and RapidArc in normal tissue sparing for nasopharyngeal carcinoma
Pubade Kaewpruk, Somvilai Chakrabandhu, Somsak Wanwilairat, Wannapha Nobnop
Journal of Radiotherapy in Practice.2020; 19(3): 237. CrossRef - Combination of Plasma MIF and VCA-IgA Improves the Diagnostic Specificity for Patients With Nasopharyngeal Carcinoma
Ning Xue, Shan Xing, Weiguo Ma, Jiahe Sheng, Zhiliang Huang, Qingxia Xu
Technology in Cancer Research & Treatment.2020; 19: 153303382093577. CrossRef - Pathological features of nasopharyngeal carcinoma: A single-center study in Vietnam
Nguyen Cuong Pham, Thanh Xuan Nguyen, Nguyen Tuong Pham, Thanh Chinh Phan, Hai Thanh Phan
Annals of Cancer Research and Therapy.2020; 28(2): 125. CrossRef - Association between variant alleles of major histocompatibility complex class II regulatory genes and nasopharyngeal carcinoma susceptibility
Ping Zhou, Sha Liu, Nan-Nan Ji, Shuang Zhang, Peng Wang, Bing Lin, Ping Yang, Xian-Tao Lin, Yi-Zheng Cai, Zi-Ming Wang, Han Zhou, Shi-Yao Sun, Xin-Bao Hao
European Journal of Cancer Prevention.2020; 29(6): 531. CrossRef - Hyperperfusion Syndrome and Baroreflex Failure following Carotid Artery Angioplasty and Stenting for Symptomatic Radiation-Associated Carotid Artery Stenosis
Hui-Meng Chang
Case Reports in Neurology.2020; 12(Suppl. 1): 76. CrossRef - Novel patterns of the Epstein-Barr nuclear antigen (EBNA-1) V-Val subtype in EBV-associated nasopharyngeal carcinoma from Vietnam
LD Thuan, ND Kha, NT Minh, LHA Thuy
Balkan Journal of Medical Genetics.2019; 22(1): 61. CrossRef - miR-29c regulates resistance to paclitaxel in nasopharyngeal cancer by targeting ITGB1
Limin Huang, Chaoquan Hu, Hui Chao, Rongpin Wang, He Lu, Hong Li, Hui Chen
Experimental Cell Research.2019; 378(1): 1. CrossRef - Systematic review and meta-analysis of prognostic microRNA biomarkers for survival outcome in nasopharyngeal carcinoma
Shanthi Sabarimurugan, Chellan Kumarasamy, Siddhartha Baxi, Arikketh Devi, Rama Jayaraj, Yukinori Takenaka
PLOS ONE.2019; 14(2): e0209760. CrossRef - Epstein‐Barr virus strain variation and cancer
Teru Kanda, Misako Yajima, Kazufumi Ikuta
Cancer Science.2019; 110(4): 1132. CrossRef - Nasopharyngeal cancer in Saudi Arabia: Epidemiology and possible risk factors
Abdullah Dakheel Alotaibi, Hussain Gadelkarim Ahmed, Abdelbaset Mohamed Elasbali
Journal of Oncological Sciences.2019; 5(1): 23. CrossRef - Association BetweenLMP-1,LMP-2, and miR-155 Expression as Potential Biomarker in Nasopharyngeal Carcinoma Patients: A Case/Control Study in Vietnam
Thuan Duc Lao, Thuy Ai Huyen Le
Genetic Testing and Molecular Biomarkers.2019; 23(11): 815. CrossRef - Lapatinib sensitivity in nasopharyngeal carcinoma is modulated by SIRT2-mediated FOXO3 deacetylation
Sathid Aimjongjun, Zimam Mahmud, Yannasittha Jiramongkol, Glowi Alasiri, Shang Yao, Ernesto Yagüe, Tavan Janvilisri, Eric W.-F. Lam
BMC Cancer.2019;[Epub] CrossRef - PPARβ/δ Agonist GW501516 Inhibits Tumorigenicity of Undifferentiated Nasopharyngeal Carcinoma in C666-1 Cells by Promoting Apoptosis
Yangyang Ji, Hui Li, Fang Wang, Linglan Gu
Frontiers in Pharmacology.2018;[Epub] CrossRef - Pembrolizumab in Asia‐Pacific patients with advanced head and neck squamous cell carcinoma: Analyses from KEYNOTE‐012
Makoto Tahara, Kei Muro, Yasuhisa Hasegawa, Hyun Cheol Chung, Chia‐Chi Lin, Bhumsuk Keam, Kenichi Takahashi, Jonathan D. Cheng, Yung‐Jue Bang
Cancer Science.2018; 109(3): 771. CrossRef - Childhood Nasopharyngeal Carcinoma (NPC): A Review of Clinical-Imaging Features and Recent Trends in Management
Mark Yoi Sun Soo
International Journal of Pediatrics and Child Heal.2018; 6: 1. CrossRef - KISS1gene suppresses metastasis of nasopharyngeal cancerviaactivation of the ERK1/2 pathway
Tingting Li, Qian Sun, Yan Zhou, Zelai He, Hao Liu, Ping Xiang, Jin Xi, Xiazi Zhang, Hao Jiang
RSC Advances.2017; 7(84): 53445. CrossRef


 PubReader
PubReader Cite
Cite
