Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 5(5); 2014 > Article
-
Original Article
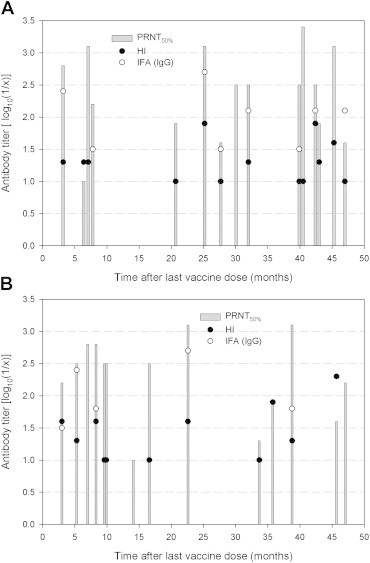
Comparison of Four Serological Tests for Detecting Antibodies to Japanese Encephalitis Virus after Vaccination in Children - Go Woon Chaa,b, Jung Eun Choa,b, Young Ran Jua,b, Young-Jin Hongc, Myung Guk Hana,b, Won-Ja Leea,b, Eui Yul Choid, Young Eui Jeonga,b,d
-
Osong Public Health and Research Perspectives 2014;5(5):286-291.
DOI: https://doi.org/10.1016/j.phrp.2014.08.003
Published online: September 6, 2014
aWHO/WPRO Japanese Encephalitis Regional Refernce Laboratory, 187 Osongsaengmyeong2-ro, Chungbuk 363-951, Korea
bDivision of Arboviruses, National Institute of Health, Korea Centers for Disease Control and Prevention, 187 Osongsaengmyeong2-ro, Chungbuk 363-951, Korea
cDepartment of Pediatrics, Inha University Medical School, Incheon, Korea
dDepartment of Biomedical Sciences, Hallym University, Chuncheon, Korea
- ∗Corresponding author. yougeui58@gmail.com
- 1Current address: Division of Medical Entomology, National Institute of Health, Korea Centers for Disease Control and Prevention, Chungbuk, Korea.
• Received: July 17, 2014 • Revised: August 23, 2014 • Accepted: August 25, 2014
© 2014 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the CC-BY-NC License (http://creativecommons.org/licenses/by-nc/3.0).
Figure & Data
References
Citations
Citations to this article as recorded by 

- Development of Colloidal Gold-Based Immunochromatographic Strips for Rapid Detection and Surveillance of Japanese Encephalitis Virus in Dogs across Shanghai, China
Dengke Zhong, Abdul Wahaab, Jiayang Zheng, Junjie Zhang, Zhiyong Ma, Jianchao Wei
Viruses.2024; 16(2): 258. CrossRef - Laboratory evaluation of ELISA and indirect immunofluorescence assay in response to emergence of Japanese encephalitis virus genotype IV in Australia
Paul Kinsella, Michael Moso, Genevieve Martin, Theo Karapangiotidis, Di Karamalakis, Suellen Nicholson, Mitch Batty, Kathy Jackson, Madeleine Marsland, Tilda Thomson, Lakshmi Manoharan, Helen O'brien, N. Deborah Friedman, Katherine Bond, Deborah A. Willia
Journal of Clinical Virology.2023; 168: 105580. CrossRef - A review on Japanese Encephalitis virus emergence, pathogenesis and detection: From conventional diagnostics to emerging rapid detection techniques
Fatima Mohsin, Shariq Suleman, Nigar Anzar, Jagriti Narang, Shikha Wadhwa
International Journal of Biological Macromolecules.2022; 217: 435. CrossRef - Recent pharmaceutical engineered trends as theranostics for Japanese encephalitis
Akshada Mhaske, Sanjiv Singh, Mohammed A.S. Abourehab, Akhilesh Kumar, Prashant Kesharwani, Rahul Shukla
Process Biochemistry.2022; 122: 115. CrossRef - Immunogenicity of a single fractional intradermal dose of Japanese encephalitis live attenuated chimeric vaccine
Luis Furuya-Kanamori, Narayan Gyawali, Deborah J Mills, Christine Mills, Leon E Hugo, Gregor J Devine, Colleen L Lau
Journal of Travel Medicine.2022;[Epub] CrossRef - In silico molecular docking in DNA aptamer development
Tholasi Nadhan Navien, Ramesh Thevendran, Hazrina Yusof Hamdani, Thean-Hock Tang, Marimuthu Citartan
Biochimie.2021; 180: 54. CrossRef - Pathobiology of Japanese encephalitis virus infection
Kiran Bala Sharma, Sudhanshu Vrati, Manjula Kalia
Molecular Aspects of Medicine.2021; 81: 100994. CrossRef - JEV-nanobarcode and colorimetric reverse transcription loop-mediated isothermal amplification (cRT-LAMP)
Gna Ahn, Se Hee Lee, Min-Suk Song, Beom-Ku Han, Yang-Hoon Kim, Ji-Young Ahn
Microchimica Acta.2021;[Epub] CrossRef - Review of Emerging Japanese Encephalitis Virus: New Aspects and Concepts about Entry into the Brain and Inter-Cellular Spreading
Filgueira, Lannes
Pathogens.2019; 8(3): 111. CrossRef - Seroprevalence of Dengue Virus Antibody in Korea
Ji Hyen Lee, Han Wool Kim, Kyung-Hyo Kim
Pediatric Infection & Vaccine.2018; 25(3): 132. CrossRef - Japanese Encephalitis: A Brief Review on Indian Perspectives
Reshma Kulkarni, Gajanan N. Sapkal, Himanshu Kaushal, Devendra T. Mourya
The Open Virology Journal.2018; 12(1): 121. CrossRef - Clinical Characteristics of Severe Japanese Encephalitis: A Case Series from South Korea
Kyung-Il Park, Manho Kim, Jun-Sang Sunwoo, Ki-Young Jung, Kon Chu, Keun-Hwa Jung, Sang Kun Lee, Soon-Tae Lee, Jangsup Moon
The American Journal of Tropical Medicine and Hygi.2017; 97(2): 369. CrossRef - JAPANESE ENCEPHALITIS, RECENT PERSPECTIVES ON VIRUS GENOME, TRANSMISSION, EPIDEMIOLOGY, DIAGNOSIS AND PROPHYLACTIC INTERVENTIONS
Arumugam Karthikeyan, Subramaniyan Shanmuganathan, Selvaraj Pavulraj, Govinthasamy Prabakar, Selvaraj Pavithra, Kannan Porteen, Govindaraj Elaiyaraja, Yashpal Singh Malik
Journal of Experimental Biology and Agricultural S.2017; 5(6): 730. CrossRef - Prevalence of Neutralizing Antibodies to Japanese Encephalitis Virus among High-Risk Age Groups in South Korea, 2010
Eun Ju Lee, Go-Woon Cha, Young Ran Ju, Myung Guk Han, Won-Ja Lee, Young Eui Jeong, Nagendra R Hegde
PLOS ONE.2016; 11(1): e0147841. CrossRef - A Novel Immunochromatographic Test Applied to a Serological Survey of Japanese Encephalitis Virus on Pig Farms in Korea
Go-Woon Cha, Eun Ju Lee, Eun-Joo Lim, Kang Suk Sin, Woo Won Park, Doo Young Jeon, Myung Guk Han, Won-Ja Lee, Woo-Young Choi, Young Eui Jeong, Lark L. Coffey
PLOS ONE.2015; 10(5): e0127313. CrossRef - Silent Circulation of Ross River Virus in French Polynesia
Maite Aubry, Jérôme Finke, Anita Teissier, Claudine Roche, Julien Broult, Sylvie Paulous, Philippe Desprès, Van-Mai Cao-Lormeau, Didier Musso
International Journal of Infectious Diseases.2015; 37: 19. CrossRef


 PubReader
PubReader Cite
Cite
