Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 9(2); 2018 > Article
-
Original Article
Foodborne Illness Outbreaks in Gyeonggi Province, Korea, Following Seafood Consumption Potentially Caused byKudoa septempunctata between 2015 and 2016 - Joon Jai Kima, Sukhyun Ryua, Heeyoung Leeb
-
Osong Public Health and Research Perspectives 2018;9(2):66-72.
DOI: https://doi.org/10.24171/j.phrp.2018.9.2.05
Published online: April 30, 2018
aDivision of Infectious Disease Control, Gyeonggi Provincial Government, Suwon, Korea
bCenter for Preventive Medicine and Public Health, Seoul National University Bundang Hospital, Seongnam, Korea
- *Corresponding author: Heeyoung Lee, Center for preventive medicine and public health, Seoul National University Bundang Hospital, Seongnam, Korea, E-mail: lhy0313@gmail.com
Copyright ©2018, Korea Centers for Disease Control and Prevention
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- Investigations into foodborne illness, potentially caused by Kudoa septempunctata, has been ongoing in Korea since 2015. However, epidemiological analysis reporting and positive K septempunctata detection in feces in Korea has been limited. The aim of this study was to provide epidemiologic data analysis of possible food poisoning caused by K septempunctata in Korea.
-
Methods
- This study reviewed 16 Kudoa outbreak investigation reports, including suspected cases between 2015 and 2016 in Gyeonggi province, Korea. Suspected Kudoa foodborne illness outbreak was defined as “evidence of K septempunctata in at least one sample.” The time and place of outbreak, patient symptoms and Kudoa (+) detection rate in feces was analyzed.
-
Results
- Kudoa foodborne illness outbreaks occurred in most patients in August (22.6%) and in most outbreaks in April (25%). The attack rate was 53.9% and the average attack rate in patients who had consumed olive flounder was 64.7%. The average incubation period was 4.3 hours per outbreak. Diarrhea was the most common symptom which was reported by 91.5% patients. The Kudoa (+) detection rate in feces was 69.2% of cases.
-
Conclusion
- Monthly distribution of Kudoa foodborne illness was different from previous studies. The Kudoa (+) detection rate in feces decreased rapidly between 25.5 and 28.5 hours of the time interval from food intake to epidemiologic survey. To identify effective period of time of investigation, we believe additional study with extended number of cases is necessary.
- Kudoa is a genus of Myxozoa which is a parasite of marine fish [1]. The parasitic symptoms of Kudoa infection are the formation of cysts viewable by the naked eye in muscles around the body, the brain, pericardium, digestive system and kidneys. Certain species of Myxozoan parasites live off muscles, forming pseudocysts, and often cause postmortem myoliquefaction in their hosts [2].
- In 2010, a new species of Myxozoa was found in farmed olive flounders from Jeju Island, Korea, and was named Kudoa septempunctata. This new species, K septempunctata, has 6 or 7 polar capsules and lives in the muscles of the olive flounders by forming pseudocysts, although this process does not cause postmortem myoliquefaction. Kudoa species cannot be seen via the naked eye and can only be diagnosed through PCR detection and microscopic tests.
- The life cycle of K septempunctata is not clear, but it is presumed that the olive flounder is infected via annelids, an intermediate host. It is likely that young, farmed flounder are infected with K septempunctata spores, which divide and multiply in number and become mature spores as the flounder grows [3].
- Japan has seen more than 100 cases of unidentifiable foodborne illness outbreaks annually since 2003. The consumption of raw olive flounders was often related to such outbreaks. Kawai et al [4] reported that K septempunctata could give rise to water and foodborne diseases, in addition to the Ministry of Health, Labour and Welfare of Japan announcing that K septempunctata was a cause of acute water and foodborne diseases in June 2011 [5].
- There are varying opinions as to whether K septempunctata causes water and foodborne diseases [6,7]. Unlike the tests carried out by Kawai et al [4] in Japan, some investigators report conflicting results [8,9]; as such, the pathogenesis of K septempunctata cannot be regarded as conclusive. However, the lack of proof of pathogenesis of K septempunctata cannot be grounds for assuming that it does not cause foodborne diseases; as such, there is a need for epidemiological investigation.
- Since 2015, the Korea National Research Institute of Health and the Institute of Health and Environment, have included tests for K septempunctata in epidemiologic investigations for suspected water and foodborne diseases [10].
- The Korea Centers for Disease Control and Prevention (KCDC), through its epidemiological investigation of infectious diseases in the Korea annual report 2015, have announced the characteristics of 11 outbreaks of K septempunctata occurring across Korea in 2015 [10]. The Guideline for Water & Foodborne Diseases Prevention and Control (2017) has classified K septempunctata infection as, “other infections,” providing details on diagnosis guidelines and various characteristics [3].
- However, as there are a limited number of existing epidemiological studies and reports on the foodborne diseases resulting from K septempunctata in Korea, the need for research on Kudoa species has become important. This study analyzes the epidemiologic characteristics of 16 outbreaks of foodborne illnesses caused by K septempunctata reported in Gyeonggi province in Korea, between 2015 and 2016. Moreover, this study examined methods to increase Kudoa (+) detection rates in feces as this had not been previously reported.
Introduction
- 1. Case criteria
- The cases of foodborne illness outbreaks potentially caused by K septempunctata were defined as cases of water and foodborne illness outbreaks where one or more patients’ human vomit or feces were found to contain K septempunctata as detected by 18S and 28S rDNA PCR [3]. Although other pathogenic organisms may have been detected, they were considered not to be the cause of the foodborne illness considering the latent period and clinical symptoms. In cases where the incubation period was short after consumption of raw fish, attempts were made to collect feces from the patients which were subject to protozoan tests, including Kudoa. Data were collected on the date of occurrence, location, number of patients and their symptoms. Kudoa detection rate in feces, and consumption rates of olive flounders in patients and non-patients were recorded and epidemiologically analyzed.
- 2. Outbreak criteria
- Report of outbreaks of water and foodborne diseases are cases defined as two or more patients who have contracted the symptoms while being connected spatially and temporally, and the causes of the symptoms are thought to be due to the same food source. Reported cases by the Gyeonggi province include cases where the source of infection is located within the province, and cases where the majority of patients reside in the province if the source of infection is unclear [3].
- This study has examined the epidemiological investigation of 16 foodborne illness outbreaks thought to be caused by K septempunctata, reported by the Gyeonggi province between January 2015 and December 2016.
- 3. Kudoa (+) detection rate in feces (%)
- For each outbreak, the Kudoa (+) detection rate in feces (%) was calculated by determining the number of Kudoa (+) specimen divided by the total number of feces specimens examined.
- Kudoa (+) detection rate in feces (%) = (Number of Kudoa (+) feces/Total Number of Feces examined) x 100
Materials and Methods
- Epidemiological analysis was carried out for a total of 16 outbreaks (4 in 2015, 12 in 2016) that had occurred in the last 2 years in Gyeonggi province (Table 1). All cases included the consumption of raw fish and related foodstuffs, including olive flounders.
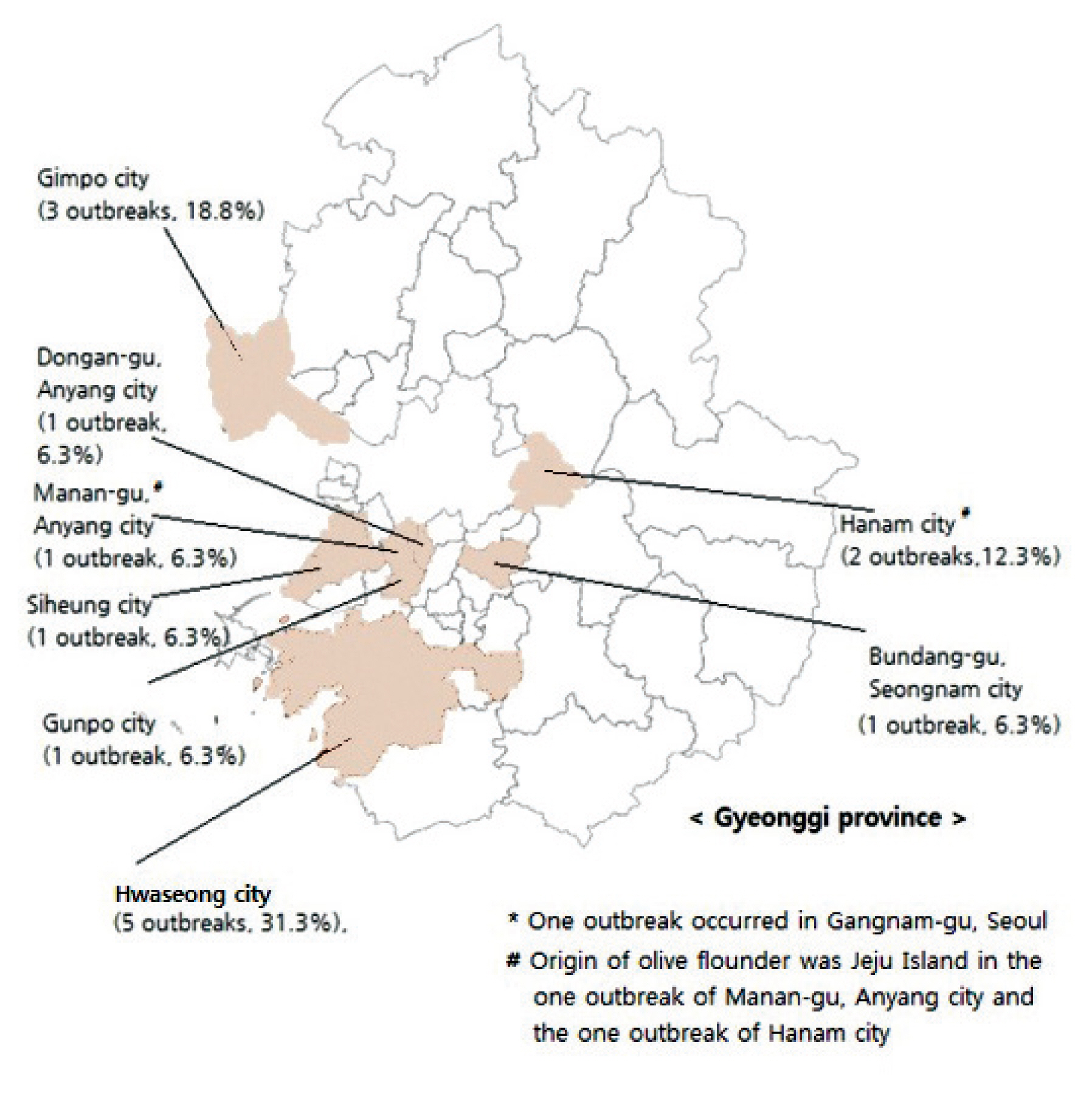
- The regional distribution of reported outbreaks of food poisoning caused by K septempunctata were: 5 outbreaks in Hwaseong city (31.3%), 3 outbreaks in Gimpo city (18.8%), 2 outbreaks in Hanam city (12.3%), and 1 outbreak each for Dongan-gu, Anyang city, Manan-gu, Anyang city, Bundang-gu, Seongnam city, Siheung city, Gunpo city and Gangnam-gu, Seoul (Figure 1).
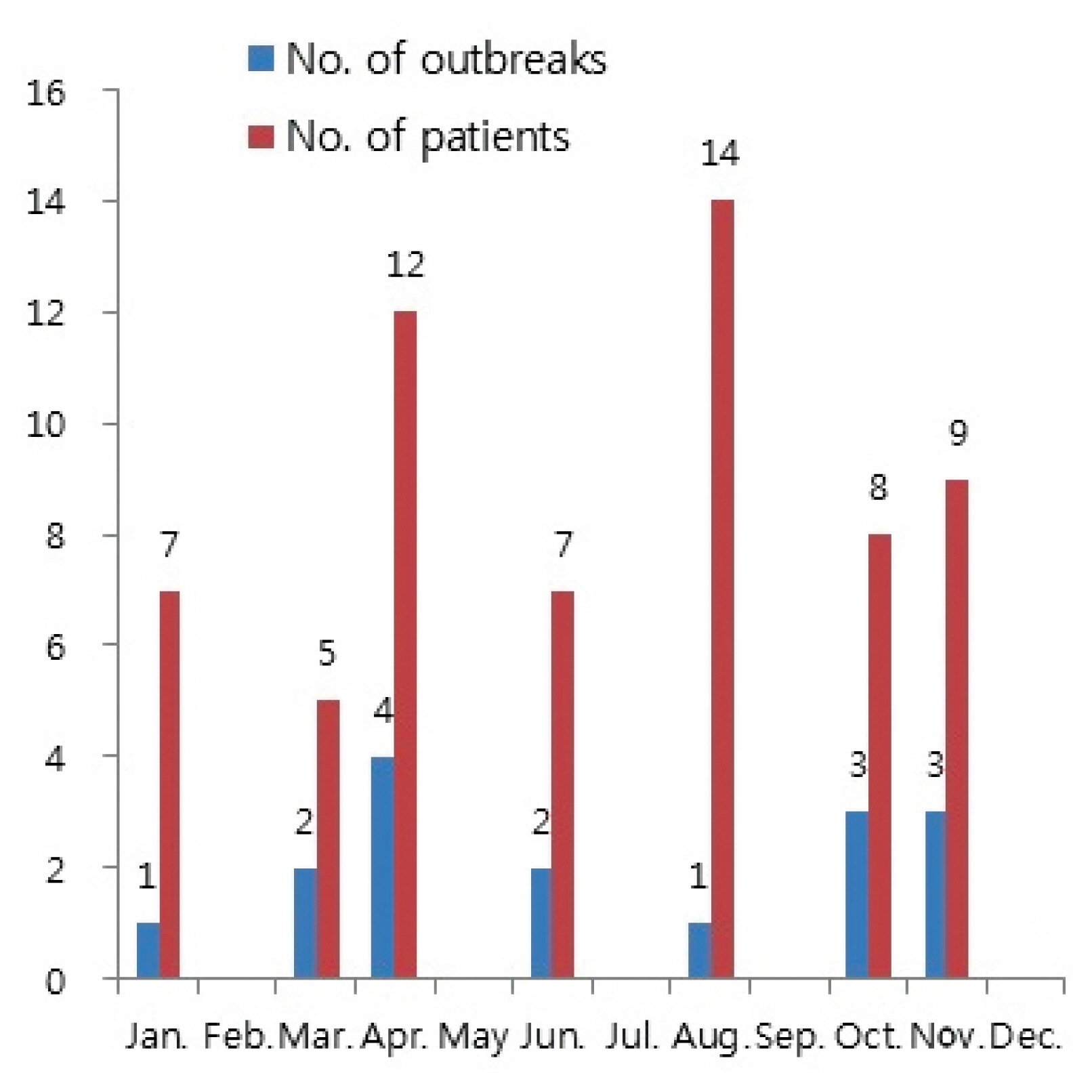
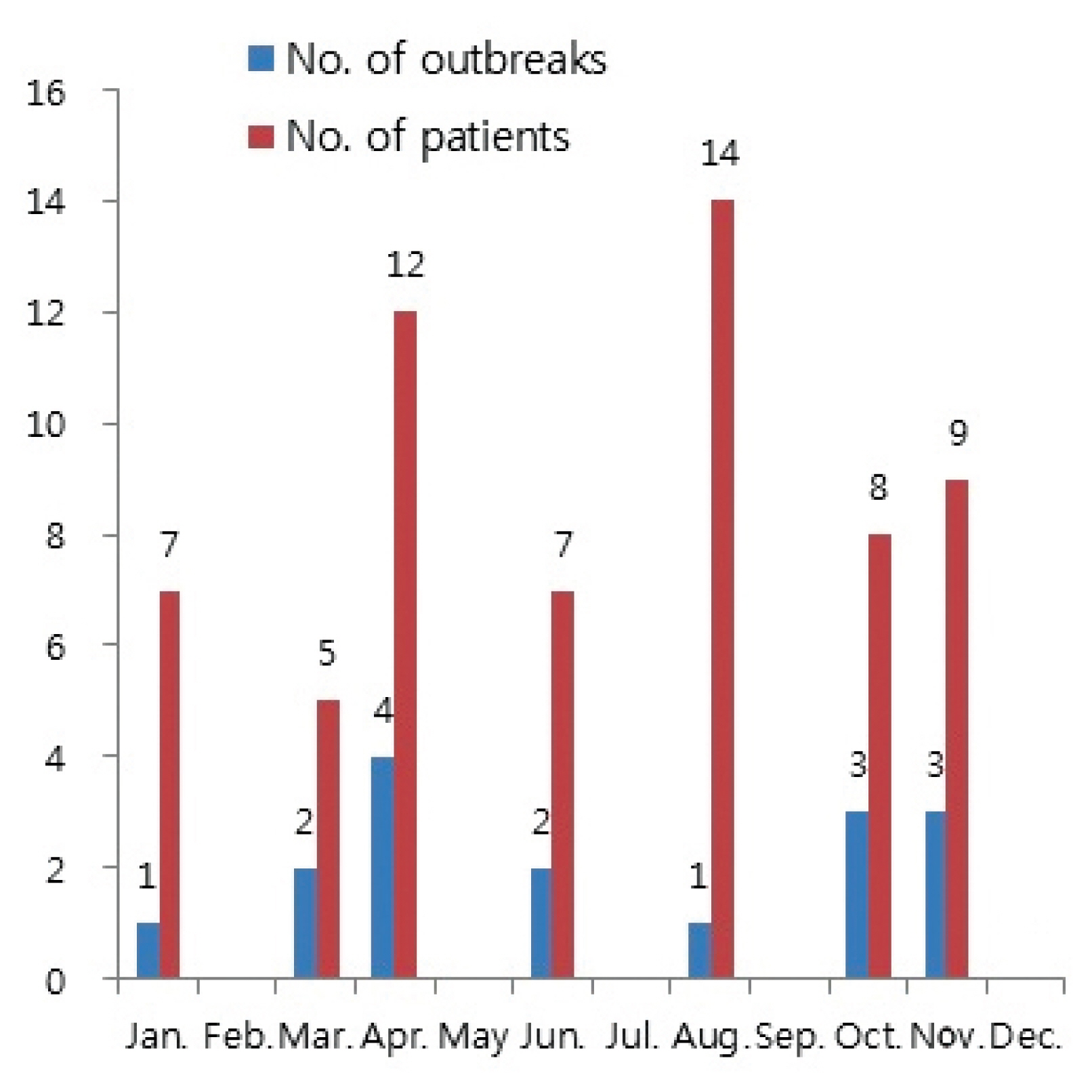
- The number of people adversely affected by K septempunctata peaked in August with 14 patients (22.6%), and April with 12 patients (19.4%).
- The number of outbreaks peaked in April with 4 outbreaks (25%), followed by October and November with 3 outbreaks (18.8%), respectively (Figure 2). The attack rate averaged 53.9%, with the rate ranging between 25% to 100% for each outbreak. The average attack rate in patients who had consumed olive flounder was 64.7%, with the rate ranging between 25% to 100% for each outbreak. The incubation period averaged 4.3 hours per outbreak, with a distribution between 2 hours to 9 hours for each patient. Patients with multiple symptoms indicated that 91.5% of patients experienced diarrhea, making it the most common symptom, followed by vomiting 86.4%, abdominal pain 67.8%, nausea 57.6% and fever 34.6%.
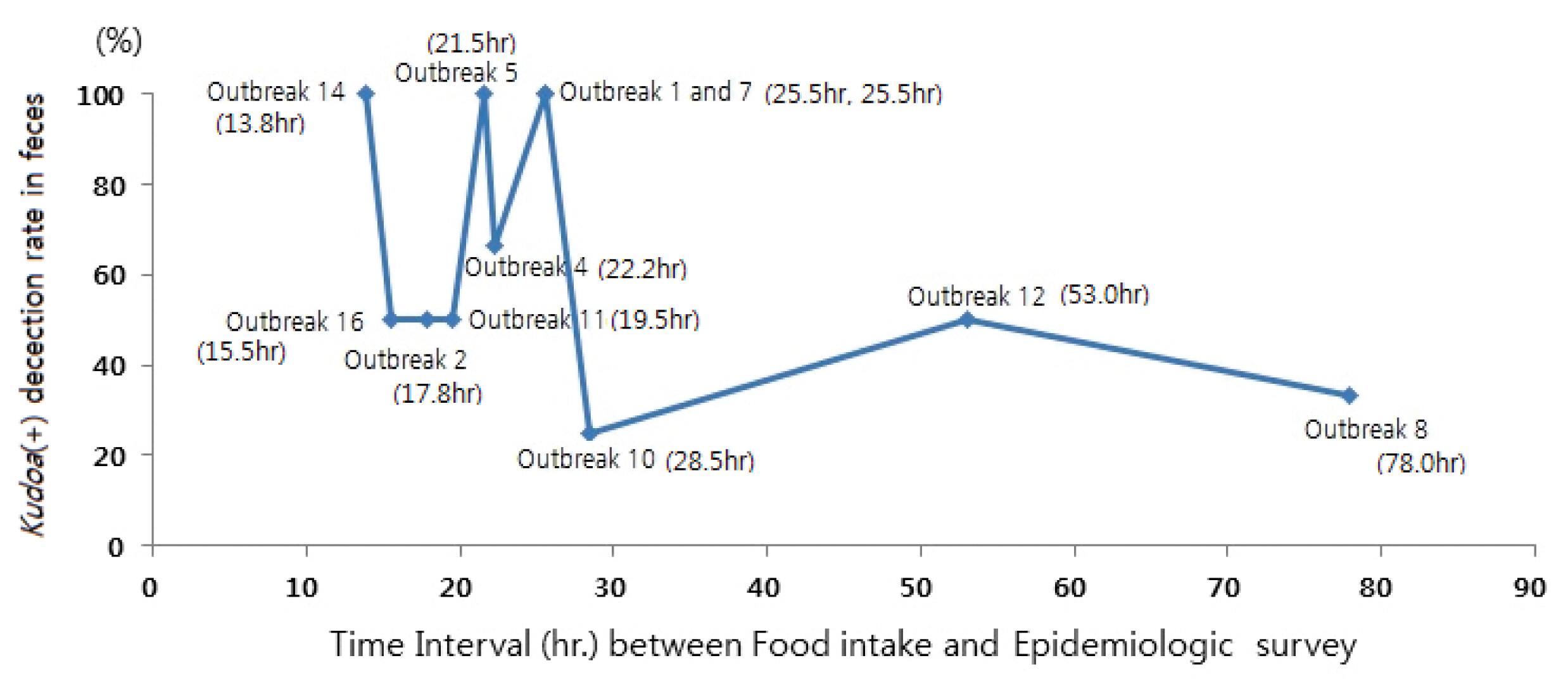
- Confirmation of Kudoa (+) detection in feces was found in 69.2% of cases, with a diverse range between 25% to 100% for each outbreak. Analyzing the outbreaks (Figure 3), Kudoa (+) detection rate in feces was much lower for patients who had more than 25.5 hours to 28.5 hours between the time of food intake and the beginning of the epidemiological investigation. The percentage of patients who consumed raw olive flounder amongst the patients was 100%, and in non-patients, the percentage was 96.8%.
Results
- The presence of Myxosporian parasites in Korea have been investigated [11], and in particular, evidence for K septempunctata infection of farmed olive flounders and their young [12, 13, 14]. In the Jeju region where olive flounders are farmed, investigators analyzed the farming environment in both Kudoa positive and negative farms [15]. Outbreaks of food poisoning caused by Kudoa in one or more patients have been investigated and reported in Korea since 2015. However, in the outbreak of water and foodborne illnesses that includes at least one case of K septempunctata in feces or vomit, it may be too hasty to judge that such a case is attributable to K septempunctata. This is because K septempunctata found in vomit or feces simply means that the patient has ingested olive flounders infected by Kudoa, and this may be independent of the occurrence of food poisoning from other pathogens [7]. The pathogenesis of the K septempunctata in animal testing has shown different results between researchers [4, 8, 9]. However, even prior to determining the pathogenesis of the K septempunctata, it is important to epidemiologically analyze cases with foodborne illness outbreaks involving patients in whom Kudoa has been detected. This study has considered the differing opinions on the pathogenesis of K septempunctata and the fact that the epidemiological investigation of infectious diseases in the Korea Annual Report 2015, has analyzed outbreaks that have involved one or more K septempunctata positive specimens. So, in this study, the possible diagnosis instead of confirmed diagnosis have been analyzed.
- In the Gyeonggi province, the mass outbreaks reported to the Comprehensive Management System at the Centre for Disease Control relating to water and foodborne diseases have reported 125 outbreaks in 2015 and 130 outbreaks in 2016; there were 48 sashimi-related outbreaks in 2015, and 52 in 2016. Amongst these foodborne outbreaks, 4 outbreaks were positive for Kudoa in 2015 and 12 in 2016. These results correlated to 3.2%, of the outbreaks reported in the province for water and foodborne illnesses in 2015, increasing to 9.2% in 2016, with 8.3% of sashimi-related outbreaks in 2015, increasing to 23.1% in 2016.
- The increase in foodborne outbreaks positive for Kudoa in 2016 compared to 2015 in Gyeonggi province may relate to the fact that public health centers have become more aware of testing for Kudoa in these outbreaks. According to the Korea National Research Institute of Health in 2015, Kudoa-related requests for testing were made for 38 outbreaks and 153 patients, nationwide; between January 1, 2016 to November 30, 2016, this increased to 77 outbreaks and 251 patients [16]. Other potential causes for the increase in incidence of Kudoa positive findings may be due to the fact that tests for Kudoa in Jeju province are not done for the entire population of olive flounders but only samples of the olive flounder, resulting in the increased risk of Kudoa positive olive flounders being consumed. Moreover, olive flounders testing positive for Kudoa cannot be exported to Japan but are available for sale domestically, and thus increasing the risk of consumption in Korea.
- The regional distribution of food does not necessarily indicate the location of origin of the raw olive flounders; as such, it is important to understand the actual source of origin of the farmed olive flounders. However, amongst the 16 outbreak reports, only Outbreak 1 and 4 were investigated to identify the source of origin, which was Jeju. Tests for origin were not completed in other outbreaks and represents a point for future improvement.
- According to the statistics of Fisheries Outlook Center of Korea Maritime Institute, the production rate of farmed flounder produced in Jeju, Wando and other regions from January 2015 to December 2016 was 67.0%, 28.8%, and 4.3%, respectively. Therefore, most of the live domestic farmed flounder originates from either Jeju or Wando. In the sashimi restaurants located in Gyeonggi province, live olive flounders are mainly supplied from Incheon Live Fish Wholesale Market, Hanam Live Fish Wholesale Market, and Noryangjin Fish Market [17]. It is presumed that the rate of the origin of live flounders supplied to the sashimi restaurants is similar. There were no related and detailed statistics on the increased outbreaks in Hwaseong city and Gimpo city in Gyeonggi province, so we could not identify their exact cause.
- Analysis of the number of outbreaks of food poisoning involving K septempunctata between 2015 and 2016 in Gyeonggi were highest in April, followed by the October and November. The national trends for outbreaks between 2015 and 2016 in Korea were 11 outbreaks in 2015 and increasing to 42 outbreaks in 2016; with May peaking with 14 outbreaks (26.4%), followed by August with 7 outbreaks (13.2%), indicating differences with the former trend [3]. In Japan, approximately 75% of all outbreaks are reported to occur between August to November[5]. In the case of Gyeonggi province, 43.9% of the outbreaks occurred between August and November which is markedly lower than the incidence in Japan, Similarly, 36.4% of outbreaks occurred in this timeframe nationally according to statistics from the Epidemiological Investigation of Infectious Diseases in the Korea Annual Report 2015 [10]. Such differences in the monthly distribution of foodborne illnesses from K septempunctata may occur from misdiagnosis of other foodborne pathogens [7] and are sometimes attributed to the differences in ocean temperature where the olive flounders are farmed [10, 18, 19, 20] Further research is required to understand the differing trends between Japan and Korea.
- Testing for Kudoa was performed on feces specimens except in Outbreak 3, where a vomit specimen tested positive for Kudoa. The fact that there were no tests for vomit specimens except in Outbreak 3, indicates that increasing future tests on vomit specimens, especially when feces specimens are not available, are required to increase the rate of diagnosis.
- In terms of the attack rate, the average attack rate in Gyeonggi province between 2015 and 2016 was 53.9%, which was lower than the national average in 2015 of 61% [10].
- The average incubation period in the past 2 years averaged 4.3 hours per outbreak (range in terms of patients: 2 hours to 9 hours). This is similar to the 2015 annual report where the average incubation period per outbreak was 4.6 hours (range in terms of patients: 1 hour to 15 hours) [10]. This is similar to the report by Yahata et al [21] of Japan (average incubation period of 5 hours) [21].
- The most common symptom was diarrhea (91.5%), followed by vomiting (86.4%), which is similar to the results from the 2015 annual report with diarrhea and vomiting, each reported at 82.9% [10]. Moreover, these results are similar to reports from Japan by Kawai [4] (diarrhea, 73.3%) or Yahata [21] (diarrhea, 80%), as they also report that diarrhea is the most common symptom.
- It was impossible to identify the source of the infection as the control groups were difficult to determine in all outbreaks, with preserved food unavailable. There were little differences between the percentage of individuals that had consumed olive flounder in the patients and non-patients (Table 1).
- K septempunctata has 3 genotypes, ST1, ST2 and ST3. Studies have shown no statistically significant differences between genotypes and the frequency of symptomatic cases and also revealed that K septempunctata found in Japan, primarily had ST1 or ST2 genotypes, whereas ST3 was commonly found in Korea in terms of regional distribution [22].
- The Korea Center for Disease Control and Prevention reported that all 84 specimens found in 45 outbreaks of K septempunctata, were related to foodborne illnesses reported between 2015 and autumn of 2016, and were all identical with the ST3 genotype [23]. The genotypes for K septempunctata found in the Gyeonggi outbreaks could not be individually reported. However, as the outbreaks in Gyeonggi province were tested by the Korea National Research Institute of Health, it was possible to determine that the the Gyeonggi outbreaks between 2015 to autumn of 2016 were all of the ST3 genotype [22, 23].
- The symptoms of K septempunctata are similar to the symptoms of other water and foodborne illnesses caused by Staphylococcus aureus or Bacillus cereus, which may lead to confusion in diagnosis [7]; as such, analysis was conducted on 2 outbreaks (Outbreaks 1 and 5) which presented the potential for coexisting infections amongst the 16 outbreaks from Gyeonggi province. In Outbreak 1, enteropathogenic Escherichia coli (EPEC) was found in 2 patients alongside Kudoa, and there was one outbreak where Giardia lamblia was detected alongside Kudoa. Considering the incubation period of Outbreak 1 ranged from 5 hours to 6.5 hours, and the patients tested positive for EPEC and G lamblia, symptoms disappeared after one day, making it logical to exclude EPEC and G lamblia as the causes of foodborne illnesses. In Outbreak 5, there was one case where Norovirus was detected along with Kudoa and considering the short incubation period (< 5 hours), it is also logical to exclude Norovirus as the cause.
- Analysis on the relationship between the time interval between food intake and epidemiologic survey and Kudoa (+) detection rate in feces, assumed that the Kudoa (+) detection rate in feces would be related to the time interval between the beginning of the symptoms and the time of feces specimen collection. However, as the time interval referred to above cannot be accurately identified through epidemiologic reports, the analysis was done with the time intervals between food intake and epidemiologic survey. Moreover, it is often impossible to obtain feces specimen from all patients in normal epidemiologic investigations of water and foodborne illnesses; as such, there was a problem of equating the Kudoa (+) detection rate in feces as being representative of the entire outbreak. Eleven relevant outbreaks were analyzed where more than 50% of the patients had feces specimen tests (Figure 3). Figure 3 shows that where the time of food consumption and beginning of the epidemiologic investigation exceeded the range of 25.5 hours to 28.5 hours, the detection rate fell rapidly; these observations indicate that to increase Kudoa (+) detection rate in feces, it is important to begin the epidemiologic investigations as soon as possible so that the time interval between food intake and epidemiologic survey is within 25.5 hours. The limitation of this research is that it has used the time interval between food exposure and the beginning of epidemiologic investigations, instead of the interval between the beginning of symptoms and feces specimen collection time, and the fact that there were insufficient number of outbreaks to power statistical analysis.
- Despite various limitations, outbreaks of foodborne illnesses involving patients testing positive for K septempunctata in Gyeonggi province has been analyzed. Given the situation where there are suspicions of increasing foodborne illnesses attributable to the K septempunctata, this study presents the need for more accurate epidemiologic investigations and specimen analysis.
Discussion
- 1. Yokoyama H, Grabner D, Shirakashi S. Transmission biology of the Myxozoa. In: Health and Environment in Aquaculture In: Carvalho E. 2012 3−42. Available from: http://www.intechopen.com/books/health-and-environment-in-aquaculture/transmission-biology-of-the-myxozoa.Article
- 2. Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y. Kudoa septempunctata n. sp. (Myxosporea: Multivalvulida) from an aquacultured olive flounder (Paralichthys olivaceus) imported from Korea. Parasitol Res 2010;107(4). 865−72. PMID: 10.1007/s00436-010-1941-8.ArticlePubMedPDF
- 3. Guideline for Water & Foodborne Diseases Prevention and Control. Osong: 2017. pp 46−337. (Korean).
- 4. Kawai T, Sekizuka T, Yahata Y, et al. Identification of Kudoa septempunctata as the causative agent of novel food poisoning outbreaks in Japan by consumption of Paralichthys olivaceus in raw fish. Clin Infect Dis 2012;54(8). 1046−52. PMID: 10.1093/cid/cir1040. PMID: 22281845.ArticlePubMedPDF
- 5. Suita-Konishi Y, Sato H, Ohnishi T. Novel foodborne disease associated with consumption of raw fish, olive flounder (Paralichthys olivaceus). Food Safety 2014;2(4). 141−50. PMID: 10.14252/foodsafetyfscj.2014026.Article
- 6. Chung YB, Bae JM. Is there evidence that Kudoa setempunctata can cause an outbreak of acute food poisoning? epiH [Intenet] 2017 39:Article ID:e2017004. 3Available from: https://doi.org/10.4178/epih.e2017004.
- 7. Lee SU. Analysis of as a cause of foodborne illness and its associated differential diagnosis. epiH 2017 39:Article ID: e2017014. 5Available from: https://doi.org/10.4178/epih.e2017014.
- 8. Ahn M, Woo H, Kang B, Jang Y, Shin T. Effect of oral administration of Kudoa septempunctata genotype ST3 in adult BALB/c mice. Parasite 2015;22:35PMID: 10.1051/parasite/2015035. PMID: 26630307. PMID: 4668110.ArticlePubMedPMC
- 9. Jang Y, Ahn M, Bang H, Kang B. Effect of Kudoa septempunctata genotype ST3 isolate from Korea on ddY suckling mice. Parasite 2016;23:18PMID: 10.1051/parasite/2016020.ArticlePubMedPMC
- 10. Chung KS. Epidemiologic investigation of infectious diseases in Korea Annual Report 2015. Osong: KCDC; 2016. pp 228−241. (Korean).
- 11. Kim Y-G, Park S-W, Choi M-C. Studies on Myxosporaridian parasites from Korean fishes. J Fish Pathol 2002;215(3). 105−10.
- 12. Song J-Y, Kim M-J, Choi H-S, Jung SH. Monitoring Kudoa septempunctata in cultured olive flounder Paralichthys olivaceus in different regions of Korea in 2013. Kor J Fish Aquat Sci 2014;47(5). 611−21.Article
- 13. Kim W-S, Kong K-H, Jung S-J, et al. A survey of Kudoa septempunctata in olive flounder (Paralichthys olivaceus) hatcheries in the southwestern coast of Korea between 2014 and 2015. J Fish Pathol 2015;28(2). 109−12. PMID: 10.7847/jfp.2015.28.2.109.ArticlePDF
- 14. Song J-Y, Choi J-H, Choi H-S, Jung SH, Park MA. Monitoring of Kudoa septempunctata in cultured olive flounder and wild fish in Jeju island during 2012. J Fish Pathol 2013;26(3). 129−37. PMID: 10.7847/jfp.2013.26.3.129.ArticlePDF
- 15. Oh H-T, Yi Y-M, Cho Y-S, Kim J-H, Lee K-H. Status of Marine Environment of Olive flounder Paralichthys olivaceus cultured in Jeju-do. JFMSE 2016;28(3). 746−59. PMID: 10.13000/JFMSE.2016.28.3.746.ArticlePDF
- 16. Cheun H. Analysis of Kudoa foodborne disease outbreaks and genotypes. Korea FETP Conference 2016. Osong: Korean Centers for Disease Control & Prevention; 2016. pp 15−23. (Korean).
- 17. Lee N-S. A Study on the Distribution and Consumption Structure of Aquacultural Flatfish. J Fish Bus Admin 2006;37(2). 61−83.
- 18. Ohnishi T, Furusawa H, Sako H, sako H, Ototake M, Hukuda Y, et al. Studies on seasonal changes in occurrence of food-borne disease associated with Kudoa septempunctata. Jpn J Food Microbiol 2013;30(2). 125−31. PMID: 10.5803/jsfm.30.125.Article
- 19. Ishmara K, Matsuura T, Tsunemoto K, Shikarakashi S. Seasonal monitoring of Kudoa yasunagai from sea water using quantitative PCR. Dis Aquat Org 2014;108:45−52. PMID: 10.3354/dao02702.ArticlePDF
- 20. Alama-Bermezo G, Sima R, Raga J, Holzer A. Understanding of myxozoan infection dynamics in the sea: Seasonality and transmission of Ceratomyxa puntazzi. Int J Parasit 2013;43:771−80. PMID: 10.1016/j.ijpara.2013.05.003.Article
- 21. Yahata Y, Sugita-Konishi Y, Ohnishi T, Toyokawa T, Nakamura N, Taniguchi K, Okabe N. Kudoa septempunctata-induced gastroenteritis in humans after flounder consumption. Jpn J Infect Dis 2015;68:119−23. PMID: 10.7883/yoken.JJID.2014.027.ArticlePubMed
- 22. Takeuchi F, Ogasawara Y, Kato K, Sekizuka T, et al. Genetic variants of Kudoa septempunctata (Myxozoa: Multivalvulida), a flounder parasite causing foodborne disease. J Fish Dis 2016;39:667−72. PMID: 10.1111/jfd.12395.ArticlePubMed
- 23. Cheun HI. Analysis on Foodborne illness cases of Kudoa septempunctata and genotyping. In: Program and abstracts of the 58th annual meeting of the Korean society for parasitology and tropical medicine; Daejeon, the Korean Society for Parasitology and Tropical Medicine; 2016. p. 21(Korean, author’s translation)..
References

| Outb No | Date of occurrence | Time interval (hr)1 | Incubation (hr) mean (min~max) | No of pts | No of persons in risk | Attack rate (%) | Diarrhea (%) | Nausea (%) | Vomiting (%) | Abdominal pain (%) | Febrile sense (%) | Feces exam rate (%)2 | Kudoa(+) rate in feces(%)3 | Attack rate with o.f.(%)4 | Intake of o.f. among pts(%)5 | Intake of o.f. among nonpts(%)6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 01/18/15 | 25.5 | 5.9 (5~6.5) | 7 | 8 | 87.5 | 71.4 | 71.4 | 57.1 | 85.7 | - | 85.7 | 100.0 | 87.5 | 100.0 | 100.0 |
| 2 | 04/19/15 | 17.8 | 3.5 (3~4) | 3 | 4 | 75.0 | 100.0 | 0 | 66.7 | 66.7 | 0 | 66.7 | 50.0 | 100.0 | 100.0 | 0 |
| 3 | 08/18/15 | -7 | 4,1 (2~9) | 14 | 18 | 77.8 | 100.0 | 57.1 | 100.0 | 42.9 | 50.0 | 21.4 | 66.7 | 100.0 | - | - |
| 4 | 10/14/15 | 22.2 | 3.5 (2~5) | 4 | 15 | 26.7 | 75.0 | 75.0 | 50.0 | 75.0 | 50.0 | 75.0 | 66.7 | 26.7 | 100.0 | 100.0 |
| 5 | 03/20/16 | 21.5 | - | 2 | 4 | 50.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 50.0 | 100.0 | 100.0 |
| 6 | 03/26/16 | - | - | 3 | 12 | 25.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 66.7 | 50.0 | 25.0 | 100.0 | 100.0 |
| 7 | 04/20/16 | 25.5 | - | 3 | 6 | 50.0 | - | - | - | - | - | 100.0 | 100.0 | 50.0 | 100.0 | 100.0 |
| 8 | 04/29/16 | 78.0 | 4.5 (3~6) | 3 | 3 | 100.0 | 100.0 | 0 | 100.0 | 0 | 0 | 100.0 | 33.3 | 100.0 | 100.0 | uncalc8 |
| 9 | 04/29/16 | 17.5 | - | 3 | 3 | 100.0 | 100.0 | 100.0 | 100.0 | 66.7 | 33.3 | 33.3 | 100.0 | 100.0 | 100.0 | uncalc |
| 10 | 06/10/16 | 28.5 | - (5~6) | 4 | 20 | 20.0 | 100.0 | 100.0 | 100.0 | 100.0 | 0 | 100.0 | 25.0 | - | - | - |
| 11 | 06/12/16 | 19.5 | - | 3 | 5 | 60.0 | 100.0 | 0 | 100.0 | 100.0 | 0 | 66.7 | 50.0 | - | - | - |
| 12 | 10/08/16 | 53.0 | 3.5 (3~4) | 2 | 2 | 100.0 | 100.0 | 100.0 | 100.0 | 50.0 | 0 | 100.0 | 50.0 | 100.0 | 100.0 | uncalc |
| 13 | 10/18/16 | 35.8 | 6.5 (6~7) | 2 | 2 | 100.0 | 100.0 | 0 | 100.0 | 50.0 | 50.0 | 50.0 | 100.0 | 100.0 | 100.0 | uncalc |
| 14 | 11/03/16 | 13.8 | - | 3 | 3 | 100.0 | 66.7 | 100 | 100.0 | 100.0 | 66.7 | 66.7 | 100.0 | 100.0 | 100.0 | uncalc |
| 15 | 11/08/16 | - | 2.5 (2~3) | 3 | 6 | 50.0 | 100.0 | 33.3 | 66.7 | 66.7 | 0 | 33.3 | 100.0 | 50.0 | 100.0 | 100.0 |
| 16 | 11/08/16 | 15.5 | 5.0 (4~6) | 3 | 4 | 75.0 | 66.7 | 0 | 66.7 | 66.7 | 0 | 66.7 | 50.0 | 75.0 | 100.0 | 100.0 |
| mean | 28.8/outb | 4.3/outb | 3.9/outb | 7.2/outb | 53.9 (62/115) | 91.5 (54/59) | 57.6 (34/59) | 86.4 (51/59) | 67.8 (40/59) | 34.6 (18/52) | 62.9 (39/62) | 69.2 (27/39) | 64.7 (55/85) | 100 (41/41) | 96.8 (30/31) |
Outb = Outbreak; No = Number; hr = hour; pts = patients; nonpts = non-patients; exam = examination; Date of occurrence = MM/DD/YY
1 Time interval (hr) = Time interval between Food intake and Epidemiologic survey
2 Feces exam rate (%) = No of Feces examination/No of patients) × 100
3 Kudoa(+) rate in Feces (%) = Kudoa(+) detection rate in Feces = (No of Kudoa(+) stool/No of Feces examination) × 100
4 Attack rate with o.f. (%) = (No of patients/No of raw olive flounder intake) × 100
5 Intake of o.f. among pts(%) = (No of raw olive flounder intake/No of patients)X 100
6 Intake of o.f. among nonpts(%) = (No of raw olive flounder intake/No of non-patients)X 100
7 - = absence of data
8 uncalc = uncalculable because No of non-patients is 0.
Figure & Data
References
Citations

- Food Safety Practices of Food Handlers in China and their Correlation with Self-reported Foodborne Illness
Yujuan Chen, Gaihong Wan, Jiangen Song, Jiajia Dai, Wei Shi, Lei Wang
Journal of Food Protection.2024; 87(1): 100202. CrossRef - Exploring the Potential Role of the Genus Kudoa (Myxosporea: Kudoidae) as an Emerging Seafood-Borne Parasite in Humans
Shokoofeh Shamsi, Diane P. Barton
Current Clinical Microbiology Reports.2024;[Epub] CrossRef - Ortholinea nupchi n. sp. (Myxosporea: Ortholineidae) from the urinary bladder of the cultured olive flounder Paralichthys olivaceus, South Korea
Sang Phil Shin, Chang Nam Jin, Hanchang Sohn, Jeongeun Kim, Jehee Lee
Parasitology International.2023; 94: 102734. CrossRef - Molecular detection and genotype analysis of Kudoa septempunctata from food poisoning outbreaks in Korea
Gyung-Hye Sung, In-Ji Park, Hee-Soo Koo, Eun-Hee Park, Mi-Ok Lee
Parasites, Hosts and Diseases.2023; 61(1): 15. CrossRef - Kudoa septempunctata Spores Cause Acute Gastroenteric Symptoms in Mouse and Musk Shrew Models as Evidenced In Vitro in Human Colon Cells
Sung-Hee Hong, Ji-Young Kwon, Soon-Ok Lee, Hee-Il Lee, Sung-Jong Hong, Jung-Won Ju
Pathogens.2023; 12(5): 739. CrossRef - Detection and characterization of Kudoa thunni from uncooked yellowfin tuna (Thunnus albacares) in Southeast Asia
Truong Dinh Hoai, Doan Thi Nhinh, Nguyen Thi Huong Giang, Saengchan Senapin, Ha Thanh Dong
Parasitology International.2022; 87: 102536. CrossRef - Descriptive study of foodborne disease using disease monitoring data in Zhejiang Province, China, 2016–2020
Xiaojuan Qi, Xialidan Alifu, Jiang Chen, Wenliang Luo, Jikai Wang, Yunxian Yu, Ronghua Zhang
BMC Public Health.2022;[Epub] CrossRef - A review of food poisoning caused by local food in Japan
Takashi Watari, Takayuki Tachibana, Azusa Okada, Kasumi Nishikawa, Kazuya Otsuki, Nobuhiro Nagai, Haruki Abe, Yasuhisa Nakano, Soshi Takagi, Yu Amano
Journal of General and Family Medicine.2021; 22(1): 15. CrossRef - Recent (2011–2017) foodborne outbreak cases in the Republic of Korea compared to the United States: a review
Sang-Oh Kim, Sang-Soon Kim
Food Science and Biotechnology.2021; 30(2): 185. CrossRef - A study on Kudoa septempunctata infection from
sashimi and sushi of olive flounder Paralichthys olivaceus in
Busan, South Korea
Hee-soo Koo, Ji-young Park, Gyung-hye Sung, Eun-hee Park, Pyeong-tae Ku, Mi-ok Lee
Fisheries and Aquatic Sciences.2021; 24(8): 277. CrossRef - Effect of environmental factors on microbiological quality of oyster farming in Amazon estuaries
Osnan Lennon Lameira Silva, Samara Maria Modesto Veríssimo, Adrianne Maria Brito Pinheiro da Rosa, Yuri Barbosa Iguchi, Emilia do Socorro Conceição de Lima Nunes, Carina Martins de Moraes, Carlos Alberto Martins Cordeiro, Diego de Arruda Xavier, Anne Suel
Aquaculture Reports.2020; 18: 100437. CrossRef - A new species Myxodavisia jejuensis n. sp. (Myxosporea: Sinuolineidae) isolated from cultured olive flounder Paralichthys olivaceus in South Korea
Sang Phil Shin, Chang Nam Jin, Han Chang Sohn, Hiroshi Yokoyama, Jehee Lee
Parasitology Research.2019; 118(11): 3105. CrossRef - Kudoa ogawai (Myxosporea: Kudoidae) Infection in Cultured Olive Flounder Paralichthys olivaceus
Sang Phil Shin, Chang Nam Jin, Han Chang Sohn, Jehee Lee
The Korean Journal of Parasitology.2019; 57(4): 439. CrossRef


 PubReader
PubReader Cite
Cite