Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 4(6); 2013 > Article
-
Original Article
Development of a Specific and Rapid Diagnostic Method for Detecting Influenza A (H1N1) pdm09 Virus Infection Using Immunochromatographic Assay - Mi Jung Jia,b, Byung Ki Choa, Young Shik Choa, Young Jin Choic, Donghyok Kwond, Kyeongcheol Shind, Joo-Yeon Leed, Chun Kangd, Byoung Su Yoonb
-
Osong Public Health and Research Perspectives 2013;4(6):342-346.
DOI: https://doi.org/10.1016/j.phrp.2013.10.006
Published online: November 7, 2013
aBiotech Laboratory, Standard Diagnostics Inc., Yongin, Korea
bDepartment of Life Science, College of Natural Science, Kyonggi University, Suwon, Korea
cDepartment of Laboratory Medicine, College of Medicine, Soonchunhyang University, Cheonan, Korea
dDivision of Influenza Virus, Korea National Institute of Health, Osong, Korea
- ∗Corresponding author. bsyoon@kyonggi.ac.kr
© 2013 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives
- The aim of this study was to develop an immunochromatographic assay (ICA) for the detection of influenza A (H1N1) pdm09 virus infection.
-
Materials and methods
- Several monoclonal antibodies against influenza A (H1N1) pdm09 virus were generated and an ICA (pdm09-ICA) was developed for the rapid and specific detection of influenza A (H1N1) pdm09 virus infection. The specificity and sensitivity of the developed assay were compared with that of hemagglutination assay and real-time reverse-transcription polymerase chain reaction (rRT-PCR).
-
Results
- The detection limit was estimated to be 1/2 (8) hemagglutinating unit; the sensitivity and specificity rates of pdm09-ICA were 75.86% (110/145) and 100% (43/43), respectively, compared with rRT-PCR. The cross-reactivity for 20 influenza viruses, including seasonal H1N1 viruses, was found to be negative except for the H1N1 virus (A/Swine/Korea/GC0503/2005).
-
Conclusion
- These results indicate that the proposed method can be easily used for rapid and specific detection of the pdm09 infection. The assay developed in this study would be a useful tool for distinguishing the pdm09 infection from seasonal influenza A and B infections.
- The influenza A (H1N1) pdm09 virus (pdm09) was first reported in the spring of 2009 in Mexico and in the United States. The virus spreads rapidly and its infection has become a world pandemic [1,2]. In August 2010, the World Health Organization (WHO) announced the postpandemic period. However, WHO recommended that the pdm09 virus is expected to continue as a seasonal virus. According to the report, younger age groups and pregnant women will continue to be affected disproportionately from the pdm09 infection [3].
- The Centers for Disease Control and Prevention recommends using the real-time reverse-transcription polymerase chain reaction (rRT-PCR) analysis for the confirmation of pdm09 infection [4]. Although rRT-PCR is a standard method and is highly sensitive and specific for the detection of influenza viruses [5–7], it requires specialized equipment, trained technicians, and takes more than 5–24 hours to generate results from clinical specimens [1,7,8]; in addition, rRT-PCR is available only in limited diagnostic laboratories [9,10]. Even though commercial influenza antigen tests can distinguish between influenza A and B viruses, they are unable to differentiate between the pdm09 and seasonal influenza A viruses [4].
- Detection of pdm09-specific hemagglutinin (HA) gene can be used for the confirmation of pdm09 virus and for differentiating it from other seasonal influenza viruses [7]. Thus, antibodies against this HA can be useful agents to develop methods that can distinguish between the pdm09 and the seasonal influenza A and B viruses.
- In this study, we have developed monoclonal antibodies against the HA of pdm09 virus and a rapid antigen test specifically to detect pdm09 based on the principle of immunochromatographic assay (pdm09-ICA).
Introduction
- 2.1 Patient and clinical specimens
- During the period from October 2009 to November 2009, two nasopharyngeal swab specimens were collected simultaneously using flocked swabs (Copan Diagnostics, Murrieta, CA, USA) from each patient (n = 188) at the Cheonan Hospital (Soonchunhyang University, Korea). One specimen was placed in a tube containing 0.3 mL of assay buffer for pdm09-ICA, and the other was placed in a tube containing 1 mL of viral transport medium for rRT-PCR.
- 2.2 Preparation of pdm09
- The pdm09 virus strain (A/Korea/01/2009) was obtained from the Korea Centers for Disease Control and Prevention. A total of 25 hemagglutinating units (HAU) of the binary ethylenimine-inactivated pdm09 were centrifuged at 4000 × g for 30 minutes at 4 °C; their supernatants were harvested and centrifuged at 70,000 × g for 3 hours at 4 °C (L8-70M; Beckman, USA). The pellets were resuspended with 1% sucrose solution, followed by ultracentrifugation on a discontinuous sucrose density gradient, as described previously [11]. Moreover, fractions that displayed hemagglutination with 1% chicken red blood cell were collected to be used as antigen in the monoclonal antibody preparation process.
- 2.3 Production of mouse monoclonal antibodies against pdm09
- Hybridoma producing mouse monoclonal antibody (MAb) against the pdm09 virus was prepared as follows: female BALB/c mice, 7 weeks of age, were intraperitoneally immunized with 200 μg of concentrated pdm09. They were emulsified in complete Freund's adjuvant (Sigma-Aldrich, St. Louis, USA) for the first immunization and in incomplete Freund's adjuvant for the second and third immunizations at 1-week intervals. Spleen cells of the immunized mice were fused with SP2/0 myeloma cells using polyethylene glycol 1500 (Roche Diagnostics GmbH, Mannheim, Germany). The hybridomas, which secrete specific antibodies against the HA of the pdm09 virus, were screened using the hemagglutination inhibition (HI) assay. Cell culture supernatants of the hybridomas were diluted in phosphate-buffered saline (pH 7.2) at a ratio of 1:1, and 22 HAU of the pdm09 cells were mixed and incubated for 30 minutes at room temperature (RT). Approximately 1% chicken red blood cell was added and the mixture was incubated for 30 minutes at RT. Finally, hybridomas that completely inhibited the hemagglutination were selected.
- In the screening test, hybridomas producing positive monoclonal antibodies were subcloned by limiting the dilution, and were grown as ascites-producing tumors in the peritoneal cavities of Freund's adjuvant-primed BALB/c mice. The monoclonal antibodies were purified from the ascites fluid by chromatography on protein G-Sepharose 4B fast flow medium (GE Healthcare Bio-Sciences, Uppsala, Sweden).
- 2.4 Development of pdm09-ICA
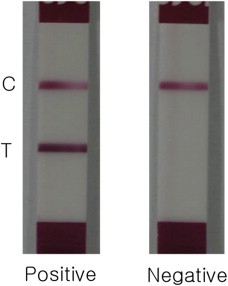
- The pdm09-ICA, which was developed for the detection of pdm09 using monoclonal antibodies, is specific to the pdm09 virus. The test strip includes a sample pad, a gold conjugate pad, a nitrocellulose membrane (Millipore Co., USA), and an absorbent pad. The captured antibodies were immobilized to two different lines on a nitrocellulose membrane, namely, the control line (1.0 mg/mL goat antimouse immunoglobulin G) and the test (2.0 mg/mL pdm09 MAb). Detector antibodies were conjugated with colloidal gold particles (Standard Diagnostic, Korea) and the gold-conjugated pdm09 MAb were dried on a glass fiber. Samples were diluted with assay buffer (0.4 M Tricine, 0.1 M NaCl, and 1% TritonX-100) in test tubes, and the test strips were added into the test tube. The pdm09 was combined with the antibody–colloidal gold conjugates, and complexes were migrated to the immobilized antibodies. The positive samples showed a purple band, whereas the negative samples did not.
- 2.5 rRT-PCR
- The viral RNA was extracted using a QIAamp viral RNA minikit (Qiagen, Germany), according to the manufacturer's instructions. Each reaction mixture for rRT-PCR was prepared according to the manufacturer's instructions, and includes 5 μL of extracted RNA and PCR reagents (Influenza A/H1N1 Detection set; Roche Applied Science, Germany). Amplification and detection were conducted on LightCycler 480 (Roche Applied Science).
Materials and methods
- 3.1 Production of anti-pdm09 monoclonal antibodies
- Mice were immunized with pdm09 emulsified with incomplete Freund's adjuvant. Spleens of mice showing seroreactivity to the pdm09 virus were isolated and fused to myeloma cells. Specific antibodies against the pdm09 virus were selected by the HI assay, which was conducted with the pdm09 and seasonal H1N1 viruses. Ten anti-pdm09 antibodies reacted with pdm09, but did not react with the seasonal H1N1. The selected antibodies were produced as ascites of BALB/c mice and purified from the ascites fluid by protein G-affinity resin. Two clones, i38 and d383, showing high reactivity toward pdm09 were selected for pdm09-ICA.
- 3.2 Development of pdm09-ICA
- These 10 antibodies were paired by negative and positive reference specimens (data not shown). The pair of i38 antibody (as capture) and d383 antibody (as detector) had different signals in the negative and positive specimens. The i38 antibody was coated on a nitrocellulose membrane as the test line, and the d383 antibody was conjugated with colloidal gold particles.
- Samples were diluted with assay buffer in test tubes. Further, the test strips were added into the test tube and the results were read for 10–15 minutes (Figure 1).
- 3.3 Performance evaluation of pdm09-ICA
- The sensitivity of the pdm09-ICA was compared with the hemagglutination assay. In brief, the hemagglutination assay was carried as follows: the pdm09 (A/Korea/01/2009) was diluted twofold in phosphate-buffered saline (pH 7.2), and 1% chicken red blood cell was added into the diluted pdm09, which was then mixed and incubated for 40 minutes at RT. To investigate the sensitivity of the pdm09-ICA, twofold dilutions of the pdm09 were made in assay buffer and applied to the test strip. The detection limit of the pdm09-ICA was estimated at 1/2 (8) HAU, and the detection limit of the hemagglutination assay was 1/2 (4) HAU (Table 1). The result of the pdm09-ICA showed relatively high sensitivity than the hemagglutination assay.
- A total of 20 influenza viruses, including the seasonal H1N1 virus, were confirmed with cross-reactivity of the pdm09-ICA for specificity of the pdm09. No positive signal was shown except for the H1N1 (A/Swine/Korea/GC0503/2005), which had a very weak positive signal (Table 2).
- We evaluated the sensitivity and specificity of the pdm09-ICA to the pdm09 using clinical specimens consisting of 145 positive and 43 negative specimens, confirmed by rRT-PCR. The sensitivity and specificity of the pdm09-ICA were 75.86% (110/145) and 100% (43/43), respectively (Table 3). The positive rates of the pdm09-ICA, according to the crossing point (CP) values of rRT-PCR, are presented in Table 4. As the CP values of rRT-PCR increases, the sensitivity of the pdm09-ICA decreases.
Results
- Influenza A viruses are made up of eight segments of encoding proteins, namely, polymerase basic proteins 1 and 2 (PB1 and PB2), polymerase acidic protein, HA, nucleoprotein (NP), neuraminidase, matrix proteins 1 and 2 (M1 and M2), and nonstructural proteins 1 and 2 [12,13]. Because HA genes are the most specific genes in the genome of the influenza A virus subtype, the methods based on viral RNA analysis target HA genes to diagnose the pdm09 [7]. However, commercially available influenza antigen tests do not distinguish the pdm09 from seasonal influenza viruses because they use antibodies against the NP of influenza A and B viruses [7,14]. For rapid and specific detection of the pdm09 among seasonal viruses, we have developed antibodies against the HA of the pdm09 and a variety of hybridoma cells, which produced HA-specific monoclonal antibodies screened by the HI assay. We selected one hybridoma producing secreted MAb that showed excellent reactivity in the HI assay.
- For the detection of seasonal influenza viruses, several commercial influenza antigen tests have been evaluated for pdm09. Compared with the rRT-PCR analysis, the sensitivity and specificity were 48.7% and 96.5% [6], 47% and 96% [15], 53.4% and 100% [14], respectively. The tests showed relatively low sensitivity for the detection of pdm09. Thus, the development of a more accurate ICA for the screening and diagnosis of pdm09 infection is urgently needed [16]. In this study, we have developed pdm09-ICA that is not only high in sensitivity but also detectable to distinguish between the pdm09 and seasonal influenza A and B viruses using monoclonal antibodies against HA of the pdm09. The pdm09-ICA developed in this study showed 75.86% sensitivity, which is higher than the sensitivity of commercial influenza antigen tests and 100% specificity compared with rRT-PCR results. When the CP value was lower than 20, the sensitivity of the pdm09-ICA was 95.45%; however, when the CP value was higher than 30, the sensitivity of the pdm09-ICA decreased to 25%. Therefore, the sensitivity of pdm09-ICA is closely related with the viral number; thus, it will be useful in the diagnosis of patients who have severe symptoms for influenza [10]. In addition, pdm09-ICA had the specificity for pdm09; 19 of the 20 different influenza viruses chosen did not show reactivity, except for H1N1 (A/Swine/Korea/GC0503/2005), which showed a very weak positive signal because the pdm09 was a result of a reassortment with swine influenza A viruses [12,13].
- The pdm 09-ICA can be easily used for rapid and specific detection of the pdm09 virus from other influenza A and B viruses, and positive signals can be visible within 10–15 minutes without using any equipment in the laboratory. This study suggests that the rapid diagnosis of the pdm09 by the pdm09-ICA might alternate with PCR, which requires equipment, cost, and time.
Discussion
-
Acknowledgements
- This work was supported by the Korea Centers for Disease Control and Prevention (Grant No. 2009-E43002-00).
Acknowledgments
-
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article information
- 1. Choi W.S., Noh J.Y., Huh J.Y.. The clinical usefulness of the SD Bioline Influenza Antigen Test® for detecting the 2009 influenza A (H1N1) virus. Yonsei Med J 52(4). 2011 Jul;683−685. PMID: 21623614.ArticlePubMed
- 2. Nougairede A., Ninove L., Zandotti C.. Point of care strategy for rapid diagnosis of novel A/H1N1 influenza virus. PLoS One 5(2). 2010 Feb;e9215PMID: 20174646.ArticlePubMed
- 3. World Health Organization. WHO recommendations for the post-pandemic period: pandemic (H1N1) 2009 briefing note 23. 2010. WHO; Geneva.
- 4. Centers for Disease Control and Prevention (CDC). Interim guidance on specimen collection, processing, and testing for patients with suspected novel influenza A (H1N1) virus infection. 2009. CDC; Atlanta.
- 5. Kang X.P., Jiang T., Li Y.Q.. A duplex real-time RT-PCR assay for detecting H5N1 avian influenza virus and pandemic H1N1 influenza virus. Virol J 7:2010 Jun;113PMID: 20515509.ArticlePubMed
- 6. Karre T., Maguire H.F., Butcher D.. Comparison of Becton Dickinson Directigen EZ Flu A+B test against the CDC real-time PCR assay for detection of 2009 pandemic influenza A/H1N1 virus. J Clin Microbiol 48(1). 2010 Jan;343−344. PMID: 19889893.ArticlePubMed
- 7. Miyoshi-Akiyama T., Narahara K., Mori S.. Development of an immunochromatographic assay specifically detecting pandemic H1N1 (2009) influenza virus. J Clin Microbiol 48(3). 2010 Mar;703−708. PMID: 20071549.ArticlePubMed
- 8. van der Vries E., Schutten M.. Satisfying the need for rapid diagnosis of new variant influenza A H1N1. Expert Rev Mol Diagn 10(3). 2010 Apr;251−253. PMID: 20370581.ArticlePubMed
- 9. Choi Y.J., Kim H.J., Park J.S.. Evaluation of new rapid antigen test for detection of pandemic influenza A/H1N1 2009 virus. J Clin Microbiol 48(6). 2010 Jun;2260−2262. PMID: 20357213.ArticlePubMed
- 10. Kim Y.K., Uh Y., Chun J.K.. Evaluation of new hemagglutinin-based rapid antigen test for influenza A pandemic (H1N1) 2009. J Clin Virol 49(1). 2010 Sep;69−72. PMID: 20663709.ArticlePubMed
- 11. Carman P.S., Povey R.C.. Comparison of the viral proteins of canine parvovirus-2, mink enteritis virus and feline panleukopenia virus. Vet Microbiol 8(5). 1983 Oct;423−435. PMID: 6316627.ArticlePubMed
- 12. Deng Y.M., Caldwell N., Barr I.G.. Rapid detection and subtyping of human influenza A viruses and reassortants by pyrosequencing. PLoS One 6(8). 2011;e23400PMID: 21886790.ArticlePubMedPMC
- 13. Trifonov V., Khiabanian H., Rabadan R.. Geographic dependence, surveillance, and origins of the 2009 influenza A (H1N1) virus. N Engl J Med 361(2). 2009 Jul 9;115−119. PMID: 19474418.Article
- 14. Kok J., Blyth C.C., Foo H.. Comparison of a rapid antigen test with nucleic acid testing during cocirculation of pandemic influenza A/H1N1 2009 and seasonal influenza A/H3N2. J Clin Microbiol 48(1). 2010 Jan;290−291. PMID: 19889892.ArticlePubMed
- 15. Diederen B.M., Veenendaal D., Jansen R.. Rapid antigen test for pandemic (H1N1) 2009 virus. Emerg Infect Dis 16(5). 2010 May;897−898. PMID: 20409404.ArticlePubMed
- 16. Louie J.K., Guevara H., Boston E.. Rapid influenza antigen test for diagnosis of pandemic (H1N1) 2009. Emerg Infect Dis 16(5). 2010 May;824−826. PMID: 20409373.ArticlePubMed
References
| Dilution (HAU) | Hemagglutination assay | pdm09-ICA |
|---|---|---|
| 1:2 (21) | + | +++ |
| 1:4 (22) | + | ++ |
| 1:8 (23) | + | ++ |
| 1:16 (24) | + | + |
| 1:32 (25) | – | + |
| 1:64 (26) | – | +w |
| 1:128 (27) | – | ± |
| 1:256 (28) | – | ± |
| 1:512 (29) | – | – |
| Real-time PCR resultsa (nb) |
No. of specimens with pdm09-ICA |
% Clinical sensitivity (95% CI) | % Clinical specificity (95% CI) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Confirmed positive (145) | 110 | 35 | 75.86 (68.29–82.1) | — | 100 | — |
| Confirmed negative (43) | 0 | 43 | — | 100 (91.8–100) | — | 55.1 |
Figure & Data
References
Citations

- Sensitive detection of influenza a virus based on a CdSe/CdS/ZnS quantum dot-linked rapid fluorescent immunochromatographic test
Anh Viet Thi Nguyen, Tung Duy Dao, Tien Thi Thuy Trinh, Du-Young Choi, Seung-Taek Yu, Hyun Park, Seon-Ju Yeo
Biosensors and Bioelectronics.2020; 155: 112090. CrossRef - Detecting Influenza A (H1N1) pdm09 Virus Infection Using Immunochromatographic Assay
Viroj Wiwanitkit
Osong Public Health and Research Perspectives.2014; 5(2): 115. CrossRef


 PubReader
PubReader Cite
Cite
