Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 4(6); 2013 > Article
-
Original Article
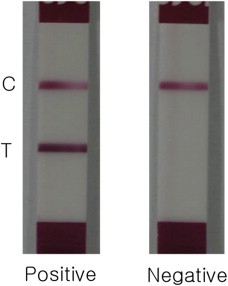
Development of a Specific and Rapid Diagnostic Method for Detecting Influenza A (H1N1) pdm09 Virus Infection Using Immunochromatographic Assay - Mi Jung Jia,b, Byung Ki Choa, Young Shik Choa, Young Jin Choic, Donghyok Kwond, Kyeongcheol Shind, Joo-Yeon Leed, Chun Kangd, Byoung Su Yoonb
-
Osong Public Health and Research Perspectives 2013;4(6):342-346.
DOI: https://doi.org/10.1016/j.phrp.2013.10.006
Published online: November 7, 2013
aBiotech Laboratory, Standard Diagnostics Inc., Yongin, Korea
bDepartment of Life Science, College of Natural Science, Kyonggi University, Suwon, Korea
cDepartment of Laboratory Medicine, College of Medicine, Soonchunhyang University, Cheonan, Korea
dDivision of Influenza Virus, Korea National Institute of Health, Osong, Korea
- ∗Corresponding author. bsyoon@kyonggi.ac.kr
• Received: October 22, 2013 • Revised: October 30, 2013 • Accepted: October 30, 2013
© 2013 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Sensitive detection of influenza a virus based on a CdSe/CdS/ZnS quantum dot-linked rapid fluorescent immunochromatographic test
Anh Viet Thi Nguyen, Tung Duy Dao, Tien Thi Thuy Trinh, Du-Young Choi, Seung-Taek Yu, Hyun Park, Seon-Ju Yeo
Biosensors and Bioelectronics.2020; 155: 112090. CrossRef - Detecting Influenza A (H1N1) pdm09 Virus Infection Using Immunochromatographic Assay
Viroj Wiwanitkit
Osong Public Health and Research Perspectives.2014; 5(2): 115. CrossRef


 PubReader
PubReader Cite
Cite
