Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 2(3); 2011 > Article
-
Articles
Cyclophilin A Cpr1 Protein Modulates the Response of Antioxidant Molecules to Menadione-induced Oxidative Stress inSaccharomyces cerevisiae KNU5377Y - Il-Sup Kima,b, Haesun Yunc, Ingnyol Jina, Ho-Sung Yoonb
-
Osong Public Health and Research Perspectives 2011;2(3):171-177.
DOI: https://doi.org/10.1016/j.phrp.2011.11.041
Published online: December 31, 2011
aDepartment of Microbiology, Kyungpook National University, Daegu, Korea
bAdvanced Bio-resource Research Center, Kyungpook National University, Daegu, Korea.
cDivision of Enteric and Hepatitis Viruses, Korea National Institute of Health, Osong, Korea.
- Corresponding author. E-mail: hyoon@knu.ac.kr
• Received: July 6, 2011 • Revised: September 27, 2011 • Accepted: October 27, 2011
Copyright ©2011, Korea Centers for Disease Control and Prevention
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License () which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,306 Views
- 14 Download
Abstract
-
Objectives
- The cellular function of cyclophilin A (CypA) differs between organisms, even though CypA is conserved in both prokaryotes and eukaryotes. The purpose of this study was to elucidate the role of activated CypA isoform CPR1 in the antioxidative mechanisms of Saccharomyces cerevisiae under menadione (MD)-induced oxidative stress.
-
Methods
- Four S. cerevisiae strains, KNU5377Y (kwt) and BY4741 (bwt), and their isogenic cpr1⊿ mutant strains (kc1 and bc1), were treated with MD, at a concentration ranging between 0.25 and 0.4 mM. Cpr1-mediated antioxidative effects were analyzed by measuring the levels of cellular glutathione (GSH) and ascorbate (AsA)-like molecules in yeast.
-
Results
- GSH and AsA-like reductant molecule concentrations were more reduced in the presence of MD in the kc1 strain than in the kwt strain; whereas, there was no significant difference between the bwt and bc1 strains under the same conditions. In kc1 strain samples, we observed a reduction in the expression of proteins related both to GSH synthesis and the recycling system, and simultaneously, downregulation of GSH synthetase and GSH reductase activities were also evident. Oxidative stress in the kc1 strain was alleviated by the application of the GSH and AsA analog.
-
Conclusion
- These results indicate that activated Cpr1 modulates the response of antioxidant molecules involved in cellular redox homeostasis of KNU5377Y during oxidative stress induced by MD.
- Oxidative stress results from the imbalance of stressful oxidants and homeostatic antioxidant systems [1]. To overcome the negative effects of transient or continuous reactive oxygen species (ROS) exposure, cells have evolved a variety of enzymatic and nonenzymatic antioxidant defense systems that are capable of removing free radicals and their byproducts to protect the integrity of cellular structures and molecular chaperone systems [1,2]. Among the many antioxidative mechanisms, oxidative stress can be buffered by the activation of chaperone-like proteins, such as cyclophilins (Cyps). Menadione (MD; 2-methyl-1,4-naphtoquinone; vitamin K3) has been used as a model for studies on oxidative stress [3].
- CypA has been isolated from a range of organisms, including bacteria and humans, and is the founding member of a class of ubiquitous and highly conserved enzymes collectively known as peptidyl cis-trans isomerases, or prolyl isomerases. These enzymes catalyze the cis-trans isomerization of the peptide bonds preceding proline residues [4]. Cyps have been reported in a wide range of metabolic processes, such as: cell division, transcriptional regulation, protein trafficking, signaling and pre-mRNA splicing [5]; molecular chaperoning, stress tolerance, receptor expression, modulation of receptor activity and, proinflammatory cytokine-like behavior [6]; bacterial effectors during animal and plant pathogenesis [7]; and cell growth, mating and virulence,and cyclosporin toxicity in the pathogenic fungus Cryp-tococcus neoformans [8]. Furthermore, Cyp expression in plant tissues increases response to different types of stress, such as heat shock and infection by pathogens [9]. In vascular smooth muscle cells, oxidative stress leads to increased expression and secretion of CypA, where the peptidyl cis-trans isomerases activity of CypA is thought to be necessary for inhibiting nitric oxide (NO)-induced apoptosis and for activating extracellular signalregulated kinase 1/2 [9]. However, the role of Cpr1 p as a component of cellular protection in yeast under
- Saccharomyces cerevisiae strains used in this study
- oxidative stress has not been extensively studied because there is no noticeable difference in the response of BY4741 wild-type and cpr1 mutant cells to H2O2 stress [10].
- In this study, we compared the concentrations of antioxidant molecules containing glutathione (GSH) and ascorbate (AsA)-like reductants in KNU5377Y and BY4741 wild-type strains and in their isogenic cpr1⊿ mutants, to investigate further the origin of the sensitivity of the cpr1⊿ mutant to MD-induced oxidative stress.
1. Introduction
Table 1.
| Strain | Genotype | Reference |
|---|---|---|
|
|
||
| KNU5377 | [12] | |
| kwt | MATa | [13] |
| kc1 (cpr1Δ) | MATa, YDR155C:: kanMX4 | [13] |
| BY4741 | ||
| bwt | MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0 | EUROSCARF |
| bc1 (cpr1Δ) | MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, YDR155C::kanMX4 | EUROSCARF |
| glr1Δ | MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0,YPL091 W::kanMX4 | EUROSCARF |
| gsh1Δ | MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0,YJL101C::kanMX4 | EUROSCARF |
| gsh2Δ | MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0,YOL049 W::kanMX4 | EUROSCARF |
| ara2Δ | MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0,YMR041C::kanMX4 | EUROSCARF |
- 2.1. Strains and growth conditions
- The strains used in this study are listed in Table 1. Precultures, grown aerobically at 30℃ overnight in YPD medium (1% yeast extract, 2% peptone, and 2% dextrose), were transferred to fresh YPD medium, and further cultured at 30℃ and shaking at 160 rpm. Cells, upon reaching the mid-log phase (OD600 = 1.5), were exposed directly to 0.4 mM MD for 1 hour at 30℃. To analyze the effects of antioxidant compounds, antioxi-dant molecules with 10 mM N-acetyl-cysteine (NAC),10 mM GSH, and 10 mM AsA were pretreated for 1 hour at 30℃. Subsequently, cells were washed twice with a phosphate-buffered saline solution (pH 7.0) to remove residual molecules. The washed cells were resuspended in YPD medium, exposed to 0.4 mM MD for 1 hour, and serially diluted to 10-4. 5 μL samples of the diluted solutions were spotted onto YPD agar plates and incubated at 30℃ for 2-3 days. In the stress sensitivity assay, the wild-type and the isogenic mutant strains, upon reaching the mid-log phase, were exposed to 0.25 mM MD for 1 hour. 5 μL samples of the diluted solutions were spotted onto YPD agar plates.
- 2.2. Determination of GSH levels
- Oxidized GSH (GSSG) and reduced GSH levels were determined by the formation of thio-nitrobenzoic acid, via the recycling assay using glutathione reductase (GR). Total glutathione content (GSH + 2 GSSG) was measured by adding either known standards (GSH) or properly diluted samples to a reaction mixture containing 0.1 M phosphate buffer (pH 7.4), 6.3 mM EDTA, 0.73 mM 5,5-dithio-bis-nitrobenzoic acid, 0.24 mM nicotinamide adenine dinucleotide phosphate, 0.09% 5-sulfosalicylic acid, and 1.2 U GR, to a final volume of 1 mL. After incubation for 20 minutes at room temperature, the sample was measured at 415 nm. An additional reaction allowed the determination of GSSG alone. In this reaction, any GSH present in the sample was first removed by vortexing for 1 hour at room temperature with 0.05% 2-vinylpyridine. After centrifugation, GSSG was measured using the same method as for total GSH measurement. The differences between total GSH equivalents (GSH + 2 GSSG) and twice the measured GSSG alone, were determined to be the measured amount of GSH in a sample [11].
- 2.3. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot analysis
- Total cellular proteins were obtained from KNU5377Y and BY4741 cells treated for 1 hour with 0.4 mM MD at 30℃ [12,13]. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed by loading 25 μg denatured proteins, and were then electrophoretically transferred to polyvinylidene fluoride membranes. The membranes were blocked and then incubated with a primary antibody for GR (Sigma, St. Louis, MO, USA), glyceraldehyde-3-phosphate dehydrogenase (Ab Frontier, Seoul, Korea), as well as with tubulin (SantaCruz Biotechnology, Santa Cruz, CA, USA) as a control. Antiglutathione synthetase (GS) antibody was synthesized with the amino acid sequence from Brassica rapa. An appropriate secondary antibody was tested, and the membranes were developed by enhanced chemiluminescence (ECL kit; GE Healthcare, Pittsburgh, PA, USA), and processed.
- 2.4. Measurement of AsA-like reductant concentration by the bipyridyl method
- Total AsA, reduced AsA, and dehydroascorbate (DHA) contents were determined using a method modified by Gillespie and Ainsworth [14]. Yeast cells were suspended in 5% (v/v) m-phosphoric acid plus glass beads (425-600 ㎛) and vortexed with a Micro-Mixer E-36 (Taitec, Tokyo, Japan). The homogenate was centrifuged at 12,000 g for 20 minutes. Total AsA content was determined in a reaction mixture consisting of 100 μL supernatant, 500 μL 150 mM KH2PO4 buffer (pH 7.4) containing 5 mM EDTA and 100 μL 10 mM dithiothreitol (DTT) to reduce DHA to AsA. The reaction mixtures were kept at room temperature for 10 minutes, after which, 100 μL 0.5% (w/v) N-ethyl-maleimide was added to the mixtures to remove excess DTT. AsA was assayed in a similar manner, except that 200 μL deionized water was substituted for DTT and N-ethylmaleimide. The colors were developed by adding reaction mixtures containing 400 μL 10% (w/v) trichloroacetic acid, 400 μL 44% (v/v) o-phosphoric acid, 400 μL α, α'-dipyridyl in 70% (v/v) ethanol, and 200 μL of 3% FeCl3. The reaction mixtures were incubated at 40℃ for 1 hour and quantified spectrophotometrically at 525 nm. The concentration of DHA was estimated from the difference in the concentrations of total AsA and reduced AsA.
- 2.5. Statistical analysis
- All experiments were repeated at least three times independently. Results were expressed as mean ± standard deviation.
2. Materials and Methods
- 3.1. Determination of GSH levels and enzyme expression, and effect of exogenous antioxidants on MD stress
- GSH is a non-protein thiol compound that is abundant in almost all aerobic organisms, and is essential for resistance and adaptation to oxidative stress [15]. To elucidate whether the acquired tolerance of the kwt strain under MD-induced oxidative stress was a result of the modulation of GSH caused by Cpr1, we measured cellular GSH content in this strain and compared it to the bwt strain under normal conditions and oxidative stress.
- The concentration of total GSH, reduced GSH and GSSG was measured in the four strains (kwt, kc1, bwt and bc1). In the kwt strain, we clearly observed an increase in total glutathione (GSH + GSSG) (Figure 1A) and reduced GSH (Figure 1B), and a decrease in GSSG (Figure 1C), which led to a large increase in the ratio of GSH to GSSG (Figure 1D), in the presence of 0.4 mM MD, compared to those concentrations exhibited by the kc1 strain. There were no differences in total GSH, GSH, and GSSG concentrations, and the GSH/GSSG ratio between the bwt and bc1 strains under the same conditions, even though the amplitude of these variations was higher in bwt and bc1 compared to that in kc1 (Figure 1).
- To examine whether the reduction of GSH levels in kc1 was a result of the malfunctioning of GSH synthesis or GSH regeneration, GS and GR activities were analyzed using immunoblotting analysis. In the kwt, oxidative stress conditions induced GS expression to a higher degree than in other strains, because GS activity in the kc1, bwt and bc1 samples could not be observed under MD-induced oxidative stress conditions. There was no difference in GR activity between the bwt and bc1 samples, although activity increased in both strains in the presence of MD (Figure 2A). Low GSH accumulation in the kc1 strain was due to either a lack of GSH synthesis by GS or of GSH recycling by GR, which resulted in increased sensitivity to MD-induced
- stress, because glr1 knockout mutants, encoding GR, and gsh2 knockout mutants, encoding GS, but not gsh1 mutants were hypersensitive to MD (Figure 2B).
- We also investigated the effects of exogenous antioxidants to determine whether the injection of GSH and NAC, a precursor of GSH synthesis, reduces sensitivity to MD-induced stress in kc1 yeast cells. As shown in Figure 2C, external supplementation with NAC and GSH alleviated stress sensitivity in all yeast strains compared to yeast strains without supplementation.
- As shown in the kc1 strain, yeast strains lacking GSH, or with their GSH altered in its redox state, are sensitive to oxidative stress induced by peroxides and superoxide anions, as well as toxic compounds that induce lipid peroxidation [15]. Exponentially growing yeast cells on rich YPD medium under normal aerobic conditions have a high redox ratio (GSH/GSSG) of 11-16.1, indicating that most of the GSH is maintained in a reduced form (GSH) (Figure 1D) [15]. Unlike in the kwt strain, exposure of kc1 cells to MD causes a reduction in the GSH/GSSG ratio and overall GSH levels (Figure 1), as well as a shift in the redox balance following an increase in the levels of protein mixed disulfides between cysteine residues and low molecular thiols (protein-S-thiolation) [16]. GSSG levels in the kwt samples were increased by 1.3-fold under oxidative
- stress conditions compared to normal conditions, even though the initial/final concentration level was 7.1-fold lower in kwt compared to kc1. This increase in GSSG concentration is consistent with the role of GSH as both an ROS scavenger and a cofactor for various antioxidant enzymes, as shown in a previous study [15]. These results show that the recycling and synthesis of GSH is probably mediated in a Cpr1-dependent manner in vivo, and is a significant mechanism of defense against MD toxicity because GSH content reduction by the repression of GS and GR in the kc1 strain increased its sensitivity to MD-induced stress.
- 3.2. Assay of intracellular AsA-like reductant and effect of exogenous addition against MD stress
- A variety of nonenzymatic and low-molecular-weight molecules, including AsA and GSH, are important defenses against the actions of ROS. Recently, it has been reported that AsA, or its analog, is capable of protecting cells against oxidative stress [17]. The intracellular concentration of AsA-like reductants was measured using the bipyridyl assay method, which is a simple technique for the analysis of AsA or AsA-like reductants. As shown in Figure 3A, there were no differences in the concentrations of AsA-like reductants in all the yeast strains under normal conditions. In the presence of MD, the concentration of reductants in the kwt samples increased approximately 1.8 times more than in the kc1 samples, even though the reductant concentrations of the kc1 strain increased under the same conditions. Unlike the kwt and kc1 strains, the levels of reductants did not differ significantly between the bwt and bc1 samples.
- A decrease in the amount of reductants present could have resulted in increased sensitivity to MD-induced stress, as in ara2 mutant cells, which biosynthesized an AsA analog called erythroascorbate (EAA; D-glycero-2-pentenono-1,4-lactone), were hypersensitive to MD exposure (Figure 3B). This led us to question whether AsA-like molecules actually provide a protective effect during oxidative stress. To estimate the antioxidative response of AsA-like reductants, yeast cells were soaked in exogenous AsA before MD exposure. Additional exogenous AsA increased MD stress tolerance in all yeast strains, particularly in kc1 (Figure 3C), indicating that AsA-like molecules such as EAA are part of the antioxidant defense system and that they are present in sufficient quantities to provide cellular protection.
- AsA is a well-known antioxidant, which acts in both plant and mammalian systems in concert with GSH to protect cells against oxidative damage by free radicals and ROS. It is well established that AsA is part of the antioxidant defense mechanism of many different cell types; however, in microorganisms, the role of AsA has been the subject of controversy. Although recent studies have reported only low levels of AsA in yeast cells,significant levels of EAA, an analog of AsA, have been detected. EAA is a five-carbon analog of ascorbic acid with an identical ring structure, and their close structural similarity has led to the suggestion that EAA might perform the same functions as AsA in organisms lacking the latter compound [18]. AsA has been shown to provide enhanced protection against oxidative stress induced by H2O2, paraquat [19-21], tert-butylhydroperoxide, cumen hydroperoxide, and MD [22] in wild-type and mutant strains of S. cerevisiae (mainly the sod⊿ and grx5⊿ mutants), and restores the reduced lifespan [23] of, or abolishes auxotrophy [24] in the sod1-deficient strain under oxidative stress. These reports uncovered a new function of AsA as a potent antioxidant in eukaryotic cells. Although yeasts synthesize EAA instead of AsA, our results show that AsA-like reductant biosynthesis is mediated by the Cpr1 protein, which presumably has an important role in cellular redox processes.
- In conclusion, our results indicate that Cpr1 protein in the yeast strain KNU5377Y, but not in BY4741, is involved in regulating the accumulation of cellular antioxidants containing GSH and AsA-like reductants under MD-induced oxidative stress. We believe further study of this model may help to elucidate the actual function of Cpr1 protein in the antioxidative process.
3. Results and Discussion
Figure 1.
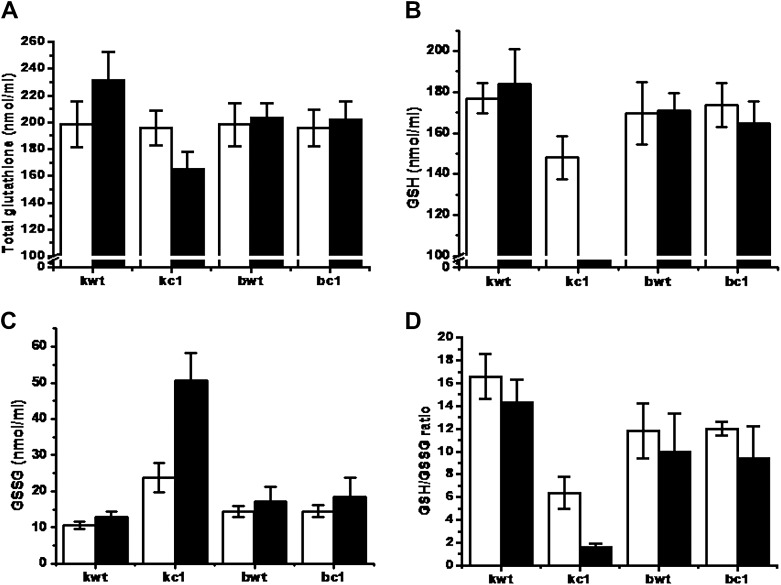
Changes in intracellular GSH contents for total GSH (A), reduced GSH (B), and GSSG (C), and the GSH/GSSG ratio (D), as measured in mid-log phase yeast cells in the absence (white bar) and presence (black bar) of 0.4 mM menadione for 1 hour. Contents are represented in nmol/109 cells/mL. GSH = reduced glutathione; GSSG = oxidized glutathione.

Figure 2.

Changes in expression of GS and GR, and the sensitivity assay of yeast strains under MD stress. Expression of GS and GR was measured using western blot analysis after exposure to 0.4 mM MD for 1 hour. The anti-tubulin antibody (Tub) was used as a housekeeping control (A). Stress sensitivity analysis of wild-type (bwt), gsh1⊿, gsh2⊿ and glr1⊿ strains of BY4741 was carried out using a spotting assay with serial dilution after 0.25 mM MD exposure for 1 hour (B). Effects of exogenous GSH and NAC were analyzed by spotting assay (C). Left, after exposure to MD without exogenous injection; middle, after exposure to MD with GSH treatment; right, after exposure to MD with NAC injection. GR = glutathione reductase; GS = glutathione synthetase; GSH = glutathione; MD = menadione; NAC = N-acetylcysteine.

Figure 3.
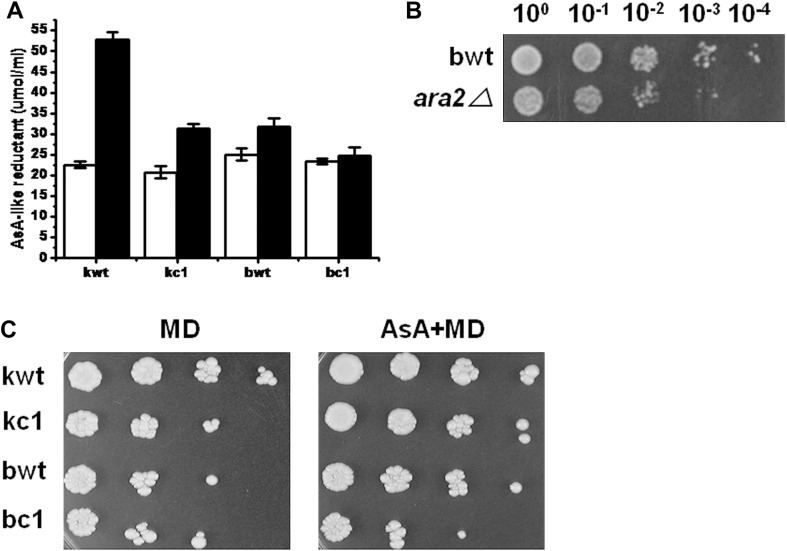
Measurement of cellular AsA-like reductant levels. AsA-like reductant levels were measured from yeast cells without (white bar) and with 0.4 mM MD (black bar) for 1 hour. The levels are represented in nmol/109 cells/mL (A). Stress sensitivity was analyzed, using the spotting assay, for the wild-type (bwt) and ara2⊿ strains exposed to 0.25 mM MD for 1 hour (B). The effect of exogenous antioxidants was analyzed using the spotting assay on yeast cells soaked in AsA for 1 hour before exposure to 0.4 mM MD for 1 hour (C). Left, after exposure to MD without exogenous stimulation; right, after exposure to MD with AsA treatment. AsA = ascorbate; MD = menadione.

-
Acknowledgements
- This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ008115), Rural Development Administration, Republic of Korea.
- 1. Jamieson DJ . Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast 12;1998;14(16). 1511−27. PMID: 9885153.ArticlePubMed
- 2. Moradas-Ferreira P Costa V . Adaptive response of the yeast Saccharomyces cerevisiae to reactive oxygen species: defences, damage and death. Redox Rep 10;2000;5(15). 277−85. PMID: 11145102.ArticlePubMed
- 3. Castro FA Herdeiro RS Panek AD et al.. Menadione stress in Saccharomyces cerevisiae strains deficient in the glutathione transferases. Biochim Biophys Acta 2;2007;1770(2). 213−20. PMID: 17157989.ArticlePubMed
- 4. Arevalo-Rodriguez M Cardenas ME Wu X et al.. Cyclophilin A and Ess 1 interact with and regulate silencing by the Sin3-Rpd3 histone deacetylase. EMBO J 7;2000;19(14). 3739−49. PMID: 10899127.ArticlePubMedPMC
- 5. Chen AP Wang GL Qu ZL et al.. Ectopic expression of ThCYP1, a stress-responsive cyclophilin gene from Thellungiella halophila, confers salt tolerance in fission yeast and tobacco cells. Plant Cell Rep 2;2007;26(2). 237−45. PMID: 16972091.ArticlePubMed
- 6. Massignan T Casoni F Basso M et al.. Proteomic analysis of spinal cord of presymptomatic amyotrophic lateral sclerosis G93A SOD1 mouse. Biochem Biophys Res Commun 2;2007;353(2). 719−25. PMID: 17196550.ArticlePubMed
- 7. Coaker G Falick A Staskawicz B . Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science 4;2005;308(5721). 548−50. PMID: 15746386.ArticlePubMed
- 8. Wang P Cardenas ME Cox GM et al.. Two cyclophilin A homologs with shared and distinct functions important for growth and virulence of Cryptococcus neoformans. EMBO Rep 6;2001;2(6). 511−8. PMID: 11415984.ArticlePubMedPMC
- 9. Perez S Weis V . Cyclophilin and the regulation of symbiosis in Aiptasia pallida. Biol Bull 8;2008;215(1). 63−72. PMID: 18723638.ArticlePubMed
- 10. Costa VM Amorim MA Quintanilha A Moradas-Ferreira P . Hydrogen peroxide-induced carbonylation of key metabolic enzymes in Saccharomyces cerevisiae: the involvement of the oxidative stress response regulators Yap1 and Skn7. Free Radic Biol Med 12;2002;33(11). 1507−15. PMID: 12446208.ArticlePubMed
- 11. Gaullier JM Lafontant P Valla A et al.. Glutathione peroxidase and glutathione reductase activities towards glutathione-derived antioxidants. Biochem Biophys Res Commun 9;1994;203(3). 1668−74. PMID: 7945316.ArticlePubMed
- 12. Kim IS Yun H Iwahashi H Jin IN . Genome-wide expression analyses of adaptive response against menadine-induced oxidative stress in Saccharomyces cerevisiae KNU5377. Process Biochem 11;2006;41(11). 2305−13.Article
- 13. Kim IS Yun H Park IS et al.. A knockout strain of CPR1 induced during fermentation of Saccharomyces cerevisiae KNU5377 is susceptible to various types of stress. J Biosci Bioeng 10;2006;102(4). 288−96. PMID: 17116574.ArticlePubMed
- 14. Gillespie KM Ainsworth EA . Measurement of reduced, oxidizedand total ascorbate content in plants. Nat Protoc 4;2007;2(4). 871−4. PMID: 17446888.ArticlePubMed
- 15. Grant CM . Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol Microbiol 2;2001;39(3). 533−41. PMID: 11169096.ArticlePubMed
- 16. Grant CM Perrone G Dawes IW . Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun 12;1998;253(3). 893−8. PMID: 9918826.ArticlePubMed
- 17. Herdeiro RS Pereira MD Panek AD Eleutherio EC . Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim Biophys Acta 3;2006;1760(3). 340−6. PMID: 16510250.ArticlePubMed
- 18. Spickett CM Smirnoff N Pitt AR . The biosynthesis of erythroascorbate in Saccharomyces cerevisiae and its role as an antioxidant. Free Radic Biol Med 1;2000;28(2). 183−92. PMID: 11281285.ArticlePubMed
- 19. Saffi J Sonego L Varela QD Salvador M . Antioxidant activity of L-ascorbic acid in wild-type and superoxide dismutase deficient strains of Saccharomyces cerevisiae. Redox Rep 8;2006;11(4). 179−84. PMID: 16984741.ArticlePubMed
- 20. Soares DG Andreazza AC Salvador M . Sequestering ability of butylated hydroxytoluene, propyl gallate, resveratrol, and vitamins C and E against ABTS, DPPH, and hydroxyl free radicals in chemical and biological systems. J Agric Food Chem 2;2003;51(4). 1077−80. PMID: 12568575.ArticlePubMed
- 21. Amari F Fettouche A Samra MA et al.. Antioxidant small molecules confer variable protection against oxidative damage in yeast mutants. J Agric Food Chem 12;2008;56(24). 11740−51. PMID: 19049288.ArticlePubMed
- 22. Lewinska A Bilinski T Bartosz G . Limited effectiveness of antioxidants in the protection of yeast defective in antioxidant proteins. Free Radic Res 11;2004;38(11). 1159−65. PMID: 15621692.ArticlePubMed
- 23. Krzepilko A Swiecilo A Wawryn J et al.. Ascorbate restores lifespan of superoxide-dismutase deficient yeast. Free Radic Res 9;2004;38(9). 1019−24. PMID: 15621721.ArticlePubMed
- 24. Zyracka E Zadrag R Koziol S et al.. Ascorbate abolishes auxotrophy caused by the lack of superoxide dismutase in Saccharomyces cerevisiae. Yeast can be a biosensor for antioxidants. J Biotechnol 2;2005;115(3). 271−8. PMID: 15639089.ArticlePubMed
Figure & Data
References
Citations
Citations to this article as recorded by 



 PubReader
PubReader Cite
Cite
