Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 2(3); 2011 > Article
-
Articles
The Emergence of Oseltamivir-Resistant Seasonal Influenza A (H1N1) Virus in Korea During the 2008-2009 Season - Woo-Young Choi, Inseok Yang, Sujin Kim, Namjoo Lee, Meehwa Kwon, Joo-Yeon Lee, Chun Kang
-
Osong Public Health and Research Perspectives 2011;2(3):178-185.
DOI: https://doi.org/10.1016/j.phrp.2011.11.042
Published online: December 31, 2011
Division of Influenza Viruses, Korea National Institute of Health, Osong, Korea.
- 1Woo-Young Choi and Inseok Yang contributed equally to this study.
- 1Woo-Young Choi and Inseok Yang contributed equally to this study.
- *Corresponding author. E-mail: ckang@nih.go.kr
• Received: July 25, 2011 • Revised: September 27, 2011 • Accepted: October 27, 2011
Copyright ©2011, Korea Centers for Disease Control and Prevention
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License () which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives
- To monitor antiviral drug resistance among seasonal influenza viruses isolated in Korea during the 2008-2009 influenza season, we examined influenza isolates collected through Korea Influenza Surveillance Scheme for antiviral drug susceptibility.
-
Methods
- For genetic analysis of antiviral drug resistance, the matrix (M2) and neuraminidase (NA) genes of each isolate were amplified by reverse transcription-polymerase chain reaction and followed by nucleotide sequencing. For phylogenetic analyses, the sequences of hemagglutinin (HA) and NA genes of each isolate were aligned using multiple alignment program. For phenotypic analysis of antiviral drug resistance, drug susceptibilities against M2 inhibitor (amantadine) and NA inhibitors (oseltavimir and zanamivir) were determined by virus yield reduction assay and fluorometric NA inhibition assay, respectively.
-
Results
- In Korea, the resistant influenza viruses against oseltamivir were first detected in sealsonal influenza A(H1N1) viruses on Week 48 of 2008. Since then, the number of oseltamivir-resistant A(H1N1) viruses was continuously increased and had reached the highest peak on Week 52 of 2008. 533 (99.8%) of 534 A(H1N1) viruses were resistant to oseltamivir and all of them harbored the H275Y mutation in the NA gene during the 2008-2009 season. The oseltamivir resistance identified by sequencing was confirmed by NA inhibition assay. Genetic analysis based on HA gene of the resistant A(H1N1) viruses revealed that the viruses were identified as A/Brisbane/10/2007-like strain which was vaccine strain for the 2008-2009 season.
-
Conclusions
- The oseltamivir-resistant A(H1N1) viruses were first emerged in Europe in November 2007 and then circulated globally. One year later, the oseltamivir-resistant A(H1N1) viruses were first detected in Korea in November 2008 and continued circulating until the Week 7 of 2009 during the 2008-2009 season. Considering the pandemic preparedness, it should be continued to monitor the emergence and the characterization of antiviral drug resistant influenza viruses.
- Influenza spreads around the world in seasonal epidemics, resulting in the deaths of 250,000-500,000 people every year [1]. Especially since April 2009, 18,449 deaths from the laboratory-confirmed pandemic (H1N1) 2009 were reported from 214 countries world-wide (as of August 1, 2010) [2]. Vaccination and antiviral treatments are essential for the prevention and control of influenza infection.
- Recently, the oseltamivir-resistant seasonal influenza A(H1N1) virus was first reported to the World Health Organization by Norway in late January 2008 [3]. However, the earliest detection of oseltamivir-resistant seasonal influenza A(H1N1) virus was in France and the United Kingdom on Week 46 of 2007 and in Norway on Week 47 of 2007 [4], and then an increased number of oseltamivir-resistant seasonal influenza A (H1N1) viruses has been spread worldwide [5-9]. According to the World Health Organization (WHO) report (as of March 18, 2009) [10], the prevalence of oseltamivir resistance in seasonal influenza A (H1N1) virus was 95% worldwide. All these oseltamivir-resistant isolates have the same mutation in the neuraminidase gene (H275Y in N1 numbering), conferring resistance to oseltamivir but not to zanamivir.
- The predominant influenza subtype circulating in Korea during 2008-2009 season was seasonal influenza A (H1N1). To determine whether the oseltamivir-resistant viruses have spread to Korea, we examined seasonal influenza A (H1N1) isolated in Korea from Week 36 of 2008 to Week 35 of 2009 for antiviral susceptibility.
- In this study, we tested 534 (18.5%, 534/2,880) influenza A (H1N1) viruses among 2880 influenza A(H1N1) viruses isolated throughout the country by the Korean Influenza Surveillance Scheme (KISS) during the 2008-2009 influenza season. For genetic analysis of M2 gene, we analyzed the amino acid substitutions at positions 26, 27, 30, 31, or 34 within the transmembrane domain of the M2 protein, known to confer resistance to amantadine [11]. For genetic analysis of NA gene, we also analyzed influenza A (H1N1) virus for amino acid substitutions at positions 119, 136, 223, 275, 293, and 295 of the NA gene (N1 numbering), known to confer resistance to neuraminidase inhibitor drugs [12,13]. We also phylogenetically analyzed both HA1 and NA sequences in the influenza A (H1N1) viruses.
- Here we described the high rate of oseltamivirresistant influenza A (H1N1) viruses identified from influenza surveillance in Korea during the 2008-2009 influenza season and the circulation of these viruses globally based on the sequence data.
1. Introduction
- 2.1. Viruses
- Influenza isolates obtained by KISS from Week 36 of 2008 to Week 35 of 2009 were used in this study. Viruses were randomly selected by region and date, and 534 A (H1N1) viruses were tested for genotype and phenotype resistant to amantadine and neuraminidase inhibitors (oseltamivir and zanamivir).
- Viruses were propagated in Madin-Darby Canine Kidney (MDCK) cells in Dulbecco’s modified essential medium (DMEM) supplemented with 5% fetal bovine serum at 35℃ and with 5% CO2. Viral stocks were stored at -70℃.
- 2.2. Extraction of viral RNA
- Viral RNA was extracted from 140 μL of virus culture using a total RNA isolation kit and a QuickGene-810 instrument (Fujifilm, Tokyo, Japan) according to the manufacturer’s instructions. RNA was eluted in 50 μL of the elution buffer provided in the kit and either used directly in reverse transcription polymerase chain reaction (RT-PCR) or stored at -70℃.
- 2.3. RT-PCR for the M2, NA, and HA genes
- Detailed methods of RT-PCR for the M2, NA, and HA genes are described in the Supplementary Methods online.
- 2.4. Sequence analysis
- The PCR products were purified for sequencing by using PCR product purification kit (Qiagen, Hilden, Germany). Both strands were sequenced by an automated method with fluorescent dideoxy-chain terminators (Applied Biosystems, Foster City, USA). At least two corresponding electropherograms for the M2, NA, and HA regions were analyzed. The sequences were aligned with the MegAlign program (DNASTAR Inc. Software, Madison, WI, USA). We analyzed the established M2 inhibitor resistance markers (26, 27, 30, 31, or 34 of M2 protein) and NA inhibitor resistance markers (119, 136, 223, 275, 293, and 295 of the NA protein in N1 numbering) in influenza viruses. We also phylogenetically analyzed the HA1 and NA coding regions in the influenza A (H1N1) virus by using MEGA with the neighbor-joining method [14]. The nucleotide sequences of the Korean isolates used in this study were compared with those of the WHO recommended vaccine strains and other reference strains from GenBank (National Center for Biotechnology Information, NCBI).
- 2.5. Neuraminidase inhibition assay
- Fluorescence-based neuraminidase inhibition (NI) assay according to the WHO Collaborating Centre for Reference and Research on Influenza manual was conducted using 405 influenza A (H1N1) viruses isolated in Korea during the 2008-2009 season. A detailed method of the neuraminidase inhibition assay is described in the Supplementary Methods online.
2. Materials and Methods
- 3.1. Seasonal surveillance
- Influenza viruses were obtained from KISS during the 2008-2009 season from influenza-like illness patients in seven cities and nine provinces, representing all regions of South Korea.
- Overall influenza activity in Korea was prevailed by A (H1N1) and A (H3N2) viruses during the 2008-2009 season compared with B virus during the 2007-2008 season. A total of 5025 influenza viruses were isolated and 3214 (64.0%), 1748 (34.8%), and 63 (1.3%) were identified as influenza A (H1N1), A (H3N2), and influenza B viruses, respectively, during the 2008-2009 season (Figure 1). The first virus in this season was isolated on Week 37 of 2008 and the strain was influenza A (H3N2) virus and then one more virus of A(H3N2) was isolated on Week 43. Influenza A (H1N1) virus was first observed on Week 48 of 2008, and it dramatically increased until Week 52 of 2008, and then decreased to Week 7 of 2009. Isolation of the viruses
- formed a peak on Week 52 of 2008. A (H3N2) viruses were rapidly increased from Week 12 and formed a peak at Week 15 of 2009. In the case of the B virus, it was first detected after Week 2 of 2009 and then several isolates were detected each week. Two major peaks of positive rate of virus isolation were observed in this season, which were directly correlated with the largest number of isolates of A (H1N1) and A (H3N2) viruses, respectively.
- 3.2. Antiviral resistance
- For genetic analysis of NA gene, nucleotide sequences of the NA gene were determined for the influenza A isolates. At the end of November 2008 (Week 48), an unusual high proportion (15/16) of oseltamivir-resistant strains was detected in A (H1N1) viruses that had an amino-acid change of histidine to tyrosine at the 275th position (N1 numbering) in the NA gene. All the resistant isolates (n = 533) possessed the H275Y mutation in the NA gene. Overall, a resistance profile for 533 of the 534 A (H1N1) viruses (99.8%) was obtained through genotypic analysis of NA genes (Table 1). Influenza A (H1N1) viruses (n = 405) were also investigated by phenotypic analysis using fluorescence-based neuraminidase inhibitor (NI) assay. Among these, all the H275Y resistant isolates (n = 404) were resistant to oseltamivir (mean IC50: 643.4), while still susceptible to zanamivir (mean IC50: 0.53). The data from NI assay correlated 100% for virus genotypes and was identified by sequencing.
- Influenza A viruses resistance to amantadine were analyzed by the sequencing of M2 region. We randomly
- Weekly distribution of oseltamivir-resistant A/H1N1 viruses
- selected 572 (17.8%) of the 3214 influenza A(H1N1) and 287 (16.4%) of the 1748 influenza A(H3N2) viruses during the 2008-2009 season. The M2 genes of 859 influenza A viruses were amplified by RT-PCR and followed by sequencing. The rate of resistance of A (H1N1) and A (H3N2) during this period was 2.1% (12/572) and 99.3% (285/287), respectively (Table 2). Our data showed that all resistant viruses contained the S31 N substitution in the M2 protein known to confer amantadine resistance [10]. Other drug-resistant M2 mutations at positions 26, 27, 30, and 34 were not detected in these resistant isolates. The amantadine resistance identified by sequencing was confirmed by virus yield reduction assay. The data from the biologic assay correlated 100% for virus genotypes identified by sequencing (data not shown).
- 3.3. Phylogenetic analysis of HA and NA genes
- A total of 523 HA (HA1 subunit 326 amino-acids) sequences (16.3%, 523/3214) and 532 NA (full-length 470 amino-acids) sequences (16.6%, 532/3214) of influenza A (H1N1) during the 2008-2009 season were analyzed to generate phylogenetic trees. The first A (H1N1) isolate during the 2008-2009 season was oseltamivir-sensitive virus and clustered in clade 2C, represented by A/Hong Kong/2652/2006, while most of
- Frequency of antiviral drug resistance of influenza A viruses during the 2008–2009 season
- the A (H1N1) viruses during the 2008-2009 season were oseltamivir-resistant viruses and belonged to clade 2B, represented by A/Brisbane/10/2007, which is the 2008-2009 influenza vaccine strain (Figure 2). The representative amino-acid changes in HA and NA genes were denoted on the phylogenetic tree (Figure 2). A/Brisbane/59/2007-like viruses (clade 2B) commonly had D35 N, R188 K, and E273 K in HA1 coding regions (H3 numbering), and H45 N, K78E, G249 K, T287I, and K329E in NA coding regions (N1 numbering) when compared with the two genes of A/New Caledonia/20/1999 vaccine strain from the 2000-2001 to the 2006-2007 seasons. Most of the isolates from the 2007-2008 season didn’t have the A189 T mutation in HA1 coding regions, while most isolates from the 2008-2009 season possessed an A189 T mutation in the HA1 coding regions (Figure 2A). Unlike the HA1 coding region, the difference in amino-acid change for each season was not observed in the NA coding region. All oseltamivir resistant viruses since the 2007-2008 season had a D354 G mutation as well as H275Y in NA coding regions compared with those of A/Brisbane/59/2007 (Figure 2B). In Korea, most A/Brisbane/59/2007-like viruses isolated from the 2008-2009 season were distinctly grouped on phylogenetic tree, which was characterized by two amino-acid changes, S141 N and G185A, in the HA genes (Figure 2A, triangle). The mutations in the HA genes were corresponded to four unique nucleotide changes: A123 G, T243A, C852 T,and A1080 G in NA genes (numbering from start codon of NA gene). This is depicted in Figure 2B (asterisk). Besides S141 N and G185A in the HA1 coding region,only a few isolates had S141 K, N183S/G185S, or G185 V/H192R variations. Of isolates containing S141 N and G185A were in the HA1 coding region, some isolates additionally harbored I184 M in the HA1 coding region and G41R or T467I in the NA coding region.
3. Results
Figure 1.
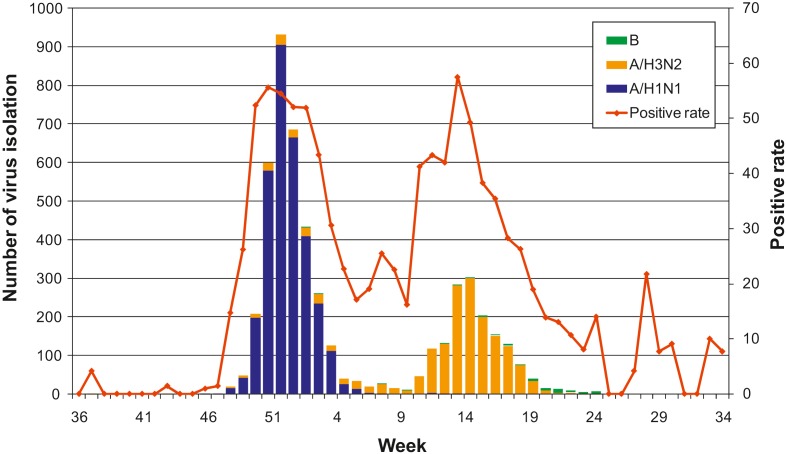
Weekly distribution of influenza virus isolation during the 2008-2009 season. Influenza A (H1N1) and A (H3N2) viruses sequentially became major isolates at initial and later times, respectively. Bars of blue, orange, and green represent number of virus isolation of A (H1N1), A (H3N2), and B viruses, respectively. The red line represents positive rate of virus isolation.

3.2.1. Resistance of NA inhibitors
3.2.2. Amantadine resistance
Table 1.
Table 2.
- To monitor the drug resistance of influenza A viruses, we performed genetic and phenotypic analyses of the NA and M2 genes of Korean isolates during the 2008-2009 season (Table 2). For oseltamivir and zanamivir resistance, a total of 829 influenza A viruses
- [534 A (H1N1) and 295 A (H3N2)] were tested. In particular, oseltamivir-resistant A (H1N1) viruses have been found through the KISS at the end of November 2008 and this is the first case of oseltamivir-resistant A (H1N1) in Korea. Until the 2007-2008 season, seasonal influenza A (H1N1), A (H3N2), and B viruses circulating in Korea remained sensitive to NA inhibitors (oseltamivir and zanamivir). Overall, 533 oseltamivirresistant A (H1N1) viruses (99.8%, 533/534) were detected during the 2008-2009 season and no oseltamivir-resistant A (H3N2) viruses (0.0%, 0/295) were observed. There were no isolates resistant to zanamivir among 829 influenza A viruses (0.0%, 0/829). For amantadine resistance, a total of 859 influenza A viruses [572 A (H1N1) and 287 A (H3N2)] were tested. The rate of resistance against amantadine of A (H1N1) and A (H3N2) was 2.1% (12/572) and 99.3% (285/287), respectively. The frequency of amantadine resistant A(H1N1) viruses (2.1%) was much lower than that of the previous season (97.8%), whereas that of amantadine resistant A(H3N2) viruses (99.3%) had the same high level seen in the previous season (100%). Based on the antiviral resistance rates of influenza A (H1N1) and A(H3N2) viruses each season, we assume that resistant viruses are circulating independently of the drug use, and the resistance rate of the isolates might depend on the degree of the transmissibility of the circulating resistant viruses, as reported previously [15].
- To investigate whether these oseltamivir resistant viruses might be transmitted from abroad, we examined travelers who suspected influenza-like illness coming through Incheon International Airport in South Korea. Of 15 specimens collected at the airport during January to March 2009, six A (H1N1) and three A (H3N2) viruses were isolated. The A (H1N1) viruses were isolated from travelers who visited Hong Kong, Kazakhstan, the Philippines, and Thailand. Through a sequence analysis of the HA and NA genes, all A (H1N1) viruses were identified as the oseltamivir resistant viruses and all A (H3N2) viruses were not. Furthermore, the sequences of these A (H1N1) viruses were grouped together with most of Korean A (H1N1) isolates (Figure 2, arrows). It revealed that the resistant A (H1N1) viruses could be introduced into Korea by travelers coming from these foreign countries.
- Based on the phylogenetic analysis of the HA and NA genes, this emergence of oseltamivir-resistant influenza A (H1N1) virus in Korea coincided with the dominant circulation of this virus during the 2007-2008 season in Europe [4]. Phylogenetic analysis of the HA1 coding region of Korean isolates during the 2008-2009 season showed most of Korean A (H1N1) viruses belonged to clade 2B, represented by A/Brisbane/10/2007, which is the 2008-2009 influenza vaccine strain (Figure 2A). Most of Korean isolates from the 2008-2009 season possessed an A189 T mutation in the HA1 coding regions (Figure 2A), as reported in Japan [5]. Phylogenetic analysis of the NA coding region of the Korean isolates during the 2008-2009 season showed that the viruses fall into clade 2B. They clustered together with A/Norway/1736/2007, which were isolated in Norway in November 2007 (Figure 2B). It also showed that all of the oseltamivir resistant viruses since the 2007-2008 season containing the Korean resistant viruses and A/Norway/1736/2007 possessed the D354 G mutation as well as the H275Y mutation in NA genes compared with those of A/Brisbane/59/2007 (Figure 2B) [4,5]. This suggests that the oseltamivir resistant viruses first emerged in Europe in November 2007 and circulated globally to many countries,including the United States, Canada, South Africa, Australia, and Japan, and then would spread to Korea in November 2008 [4-9]. These oseltamivir-resistant viruses are easily transmissible and circulate independently of drug use. It suggests that the genetic variations of the HA and NA genes may contribute to their overall fitness and transmissibility.
- Here, our data provide the information on the adequate use of antiviral drugs in a rapid manner for influenza treatment through antiviral resistance monitoring. Thorough monitoring of antiviral resistance in influenza viruses is therefore an essential part of influenza virus surveillance and is needed to track the emergence and worldwide spread of drug-resistant viruses.
- Supplementary data
- Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.phrp.2011.11.042.
4. Discussion
Figure 2.
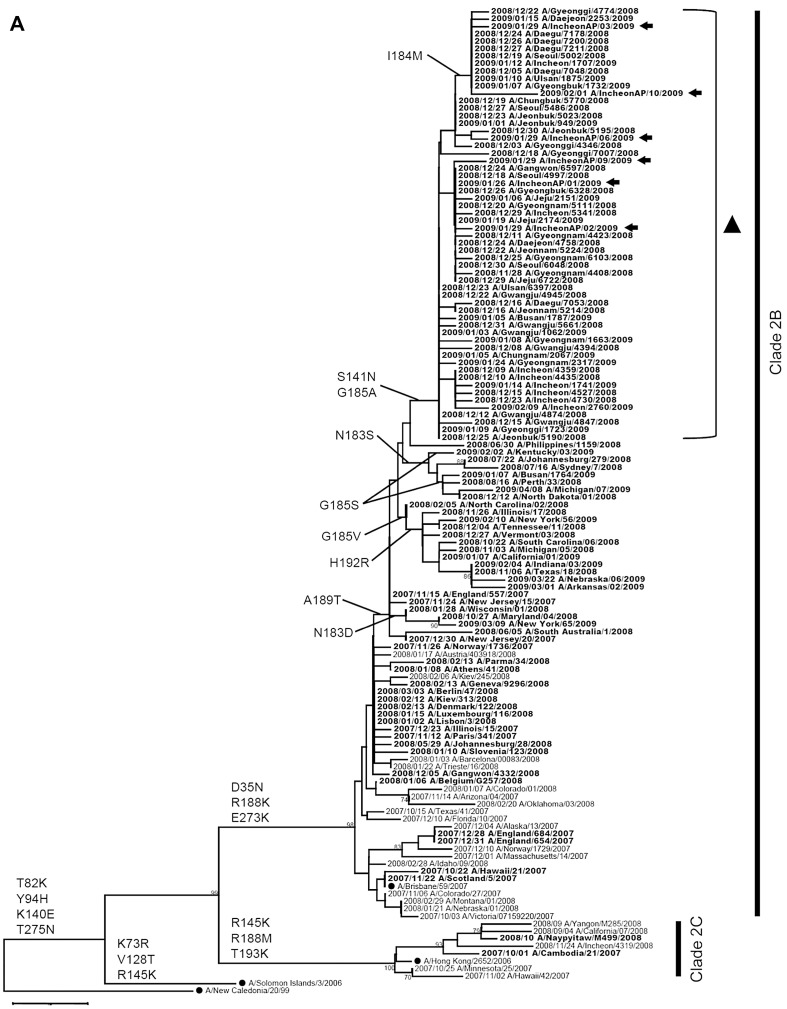
Phylogenetic tree analysis of the HA1 coding region of the (A) hemagglutinin; and (B) neuraminidase coding region of human influenza A (H1N1) viruses isolated in Korea during the 2008-2009 season. Phylogenetic tree analysis of the HA1 and NA coding regions from strains isolated in Korea, rooted with A/New Caledonia/20/1999. Oseltamivir-resistant strains are denoted in bold.

-
Acknowledgements
- This study was supported by an intramural research fund from the Korea National Institute of Health (No.4838-304-210-11). We thank 16 Public Institutes of Health and Environmental Research for the diagnosis of influenza virus and sentinel clinic doctors for collecting specimens.
- 1. World Health Organization.Influenza (Seasonal) 2009 Availableonline: http://www.who.int/mediacentre/factsheets/fs211/en. [date accessed 7 June 2011]..
- 2. World Health Organization.Pandemic (H1N1) 2009-update 112. 2010 Available online: http://www.who.int/csr/don/2010_08_06/en/index.html. [date accessed 7 June 2011].
- 3. World Health Organization. Influenza A(H1N1) virus resistance to oseltamivir: last quarter 2007 to first quarter 2008. 2008 Jun 13 [cited 2008 Jul 11]. 2008 Available online: http://www.who.int/csr/disease/influenza/oseltamivir_summary/en/index.html. [date accessed 7 June 2011].
- 4. Meijer A Lackenby A Hungnes O et al.. Oseltamivir-resistant influenza virus A(H1N1), Europe, 2007-08 season. Emerg Infect Dis 4;2009;15(4). 552−60. PMID: 19331731.ArticlePubMedPMC
- 5. the Japanese Influenza Collaborative Study Group.Baranovich T Saito R Suzuki Y et al.. Emergence of H274Y oseltamivirresistant A(H1N1) influenza viruses in Japan during the 2008-2009 season. J Clin Virol 1;2010;47(1). 23−8. PMID: 19962344.ArticlePubMed
- 6. Besselaar TG Naidoo D Buys A et al.. Widespread oseltamivr resistance in influenza A viruses (H1N1), South Africa. Emerg Infect Dis 11;2008;14(11). 1809−10. PMID: 18976580.ArticlePubMedPMC
- 7. Dharan NJ Gubareva LV Meyer JJ et al.. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 3;2009;301(10). 1034−41. PMID: 19255110.ArticlePubMed
- 8. Hurt AC Ernest J Deng YM et al.. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antivir Res 7;2009;83(1). 90−3. PMID: 19501261.ArticlePubMed
- 9. Lackenby A Hungnes O Dudman SG et al.. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Eur Surveill 1;2008;13(5). 1−2.Article
- 10. World Health Organization. Influenza A(H1N1) virus resistance to oseltamivir-2008/2009 influenza season, northern hemisphere: fourth quarter 2008 to 31 January 2009. 2009 Mar 18 [cited 2009 Mar 21]. 2009 Available online: http://www.who.int/csr/disease/influenza/H1N1webupdate20090318%20ed_ns.pdf. [dateaccessed 7 June 2011].
- 11. Abed Y Goyette N Boivin G . Generation and characterization of recombinant influenza A(H1N1) viruses harboring amantadine resistance mutations. Antimicrob Agents Chemother 2;2005;49(2). 556−9. PMID: 15673732.ArticlePubMedPMC
- 12. Abed Y Baz M Biovin G . Impact of neuraminidase mutations conferring influenza resistance to neuraminidase inhibitors in the N1 and N2 genetic backgrounds. Antivir Ther 2006;11(8). 971−6. PMID: 17302366.ArticlePubMedPDF
- 13. Yen HL Hoffmann E Taylor G et al.. Importance of neuraminidase active-site residues to the neuraminidase inhibitor resistance of influenza viruses. J Virol 9;2006;80(17). 8787−95. PMID: 16912325.ArticlePubMedPMC
- 14. Tamura K Dudley J Nei M Kumar S . MEGA4: molecular evolution genetics analysis (MEGA) software version 4.0. Mol Biol Evol 8;2007;24(8). 1596−9. PMID: 17488738.ArticlePubMed
- 15. Choi WY Kim SJ Lee NJ et al.. Amantadine-resistant influenza A viruses isolated in South Korea from 2003 to 2009. Antiviral Res 11;2009;84(2). 199−202. PMID: 19720085.ArticlePubMed
Figure & Data
References
Citations
Citations to this article as recorded by 

- Pharmacokinetics and safety of a novel influenza treatment (baloxavir marboxil) in Korean subjects compared with Japanese subjects
Yun Kim, Sangwon Lee, Yohan Kim, In‐Jin Jang, SeungHwan Lee
Clinical and Translational Science.2022; 15(2): 422. CrossRef - 2018–2019 antiviral drug sensitivity of the influenza virus strains isolated from various regions of Kazakhstan
T. I. Glebova, N. G. Klivleyeva, G. V. Lukmanova, N. T. Saktaganov, A. M. Baimukhametova
Russian Journal of Infection and Immunity.2021; 11(6): 1159. CrossRef - Assessment of Intensive Vaccination and Antiviral Treatment in 2009 Influenza Pandemic in Korea
Chaeshin Chu, Sunmi Lee
Osong Public Health and Research Perspectives.2015; 6(1): 47. CrossRef - Doing Mathematics with Aftermath of Pandemic Influenza 2009
Hae-Wol Cho, Chaeshin Chu
Osong Public Health and Research Perspectives.2015; 6(1): 1. CrossRef - Antiviral treatment of influenza in South Korea
Young June Choe, Hyunju Lee, Hoan Jong Lee, Eun Hwa Choi
Expert Review of Anti-infective Therapy.2015; 13(6): 741. CrossRef - Synthesis and anti-influenza virus activity of 4-oxo- or thioxo-4,5-dihydrofuro[3,4-c]pyridin-3(1H)-ones
Ye Jin Jang, Raghavendra Achary, Hye Won Lee, Hyo Jin Lee, Chong-Kyo Lee, Soo Bong Han, Young-Sik Jung, Nam Sook Kang, Pilho Kim, Meehyein Kim
Antiviral Research.2014; 107: 66. CrossRef - Was the Mass Vaccination Effective During the Influenza Pandemic 2009–2010 in Korea?
Hae-Wol Cho, Chaeshin Chu
Osong Public Health and Research Perspectives.2013; 4(4): 177. CrossRef - How to Manage a Public Health Crisis and Bioterrorism in Korea
Hae-Wol Cho, Chaeshin Chu
Osong Public Health and Research Perspectives.2013; 4(5): 223. CrossRef - Generation and Characterization of Recombinant Influenza A(H1N1) Viruses Resistant to Neuraminidase Inhibitors
WooYoung Choi, Jin-Young Shin, Hwan-Eui Jeong, Mi-Jin Jeong, Su-Jin Kim, Joo-Yeon Lee, Chun Kang
Osong Public Health and Research Perspectives.2013; 4(6): 323. CrossRef - Occurrence and characterization of oseltamivir-resistant influenza virus in children between 2007-2008 and 2008-2009 seasons
Seoung Geun Kim, Yoon Ha Hwang, Yung Hae Shin, Sung Won Kim, Woo Sik Jung, Sung Mi Kim, Jae Min Oh, Na Young Lee, Mun Ju Kim, Kyung Soon Cho, Yeon Gyeong Park, Sang Kee Min, Chang Kyu Lee, Jun Sub Kim, Chun Kang, Joo Yeon Lee, Man Kyu Huh, Chang Hoon Kim
Korean Journal of Pediatrics.2013; 56(4): 165. CrossRef


 PubReader
PubReader Cite
Cite
