Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 13(6); 2022 > Article
-
Brief Report
A low risk of nosocomial transmission of subclinical tuberculosis to neonates in a postpartum care center under COVID-19 control measures -
In Kyoung Kim1
 , So Jung Kim1
, So Jung Kim1 , Kyoung Hee Bae1
, Kyoung Hee Bae1 , Mi Young Kim2
, Mi Young Kim2 , Ji Eun Oh3
, Ji Eun Oh3 , Mi Gyeong Lee3
, Mi Gyeong Lee3 , Young Ae Kang4
, Young Ae Kang4 , Jin Su Song1,5
, Jin Su Song1,5
-
Osong Public Health and Research Perspectives 2022;13(6):448-452.
DOI: https://doi.org/10.24171/j.phrp.2022.0235
Published online: December 16, 2022
1Division of Infectious Disease Response, Capital Regional Center for Disease Control and Prevention, Seoul, Korea
2Gimhae Airport National Quarantine Station, Busan, Korea
3Division of Public Health Administration, Suji-gu Public Health Center, Yongin, Korea
4Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
5Graduate School of Global Development and Entrepreneurship, Handong Global University, Pohang, Korea
- Corresponding author: Jin Su Song Graduate School of Global Development and Entrepreneurship, Handong Global University, 558 Handong-ro, Heunghae-eup, Buk-gu, Pohang 37554, Korea E-mail: dicaful@handong.ac.kr
© 2022 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 2,112 Views
- 98 Download
Abstract
- We report the results of investigating and managing a tuberculosis (TB) exposure in a postpartum care center. Among the contacts exposed to a nursing assistant with subclinical TB, 5 of 44 neonates (11.4%) had positive tuberculin skin tests (TSTs) at 3 months of age, and all the TST-positive neonates received the Bacille Calmette-Guérin vaccination. Seven of 28 healthcare workers (25.0%) and 1 of 3 household contacts (33.3%) were positive in the initial or repeated interferon-gamma release assay. None of the contacts developed TB disease during the study period. Annual TB examinations of healthcare personnel at a postpartum care center under the Tuberculosis Prevention Act in South Korea enabled the early detection of subclinical TB, which reduced the risk of transmission to neonates under strict coronavirus disease 2019 prevention measures.
- The nosocomial transmission of tuberculosis (TB) to neonates is a recognized risk factor with a high mortality rate [1]. The growth in the number of private postpartum care centers in South Korea, which provide customized services for mothers and their newborns during the postpartum period, has contributed to increased TB exposure to neonates, causing serious public concerns [2]. However, uncertainty about the risk of TB transmission to neonates makes it challenging to determine the appropriate level of TB exposure management. This study evaluated the risk of TB transmission among the contacts exposed to a nursing assistant with subclinical TB at a postpartum care center.
Introduction
- Index Case
- A 58-year-old nursing assistant working at a postpartum care center was referred to a tertiary university-affiliated hospital following abnormal findings on chest radiography (CXR), performed as part of an annual TB screening program on August 9, 2021. Subsequently, computed tomography and repeated CXR showed a nodule in the right upper lung, but no cavities. Bronchoalveolar lavage fluid analysis revealed smears for acid-fast bacilli (AFB), and cultures in liquid and solid media for Mycobacterium tuberculosis were negative; however, polymerase chain reaction for M. tuberculosis was positive. Additionally, the Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) assay was positive for M. tuberculosis complex without rifampin resistance. The patient had no symptoms such as fever, cough, or sputum production. In 2017, an interferon-gamma release assay (IGRA) was negative, and a CXR during a 2020 recruitment check-up was normal. The index case worked 8 hours per day, 4 to 5 days a week, at the facility. She spent around 6 hours caring for newborns, such as feeding, soothing, and changing diapers in a neonate care room, while always using a facial mask. On average, she cared for 3.5 neonates per day. Casual contacts or conversations between the index case and other nursing staff may have occurred in a dressing room or during 30 minutes of duty handover.
- Setting
- The postpartum care center was a 4-story structure with a total area of 657 m2. The mothers resided in 19 separate rooms on the first and second floors. The neonatal care unit was located on the third floor, which measured 59.2 m2, and was divided into 3 sections: a nursing room (10.3 m2), an intensive care room (15.9 m2), and a neonate care room with 19 newborn cribs (33.0 m2). The building was served by a mechanical ventilation system, and each room was equipped with a portable air cleaner appliance. In accordance with the national coronavirus disease 2019 (COVID-19) response protocol, mandatory face mask-wearing and respiratory hygiene were strictly followed in the entire facility, and visitors were not allowed except for the mother’s spouse.
- Contact Investigation
- According to the Korea Disease Control and Prevention Agency guidelines [3], the beginning of the infectious period was determined to be 4 weeks before the date of suspected diagnosis, given that the index case had no TB symptoms and was sputum smear-negative in AFB without lung cavities on CXR. Given the index case’s work schedule and absence in the facility, the infectious period was estimated as July 13 to August 7, 2021. All contacts, including neonates, healthcare workers (HCWs), and household contacts, were examined and followed. The exposed neonates underwent CXRs and were recommended to receive isoniazid prophylaxis (10 mg/kg/day) until 3 months. Tuberculin skin tests (TSTs) using 2 Tuberculin Unit (TU) of Purified Protein Derivative (PPD) RT23 (Statens Serum Institut, Copenhagen, Denmark) were performed using the Mantoux method at the end of preventive TB treatment. A positive TST was defined as an induration of ≥5 mm in neonates without Bacille Calmette-Guérin (BCG) vaccination and ≥10 mm in those with BCG vaccination. HCWs and household contacts were initially offered CXRs and IGRAs. After 8 weeks, they underwent second CXRs, and those with negative results at the initial IGRA were offered repeat IGRAs. Along with the findings of the epidemiological investigation, the Korean National TB Surveillance System (KNTSS) database was used to verify previous TB history, latent tuberculosis infection (LTBI) test results, and treatment outcomes. All contacts were followed up for around a year after the last exposure to the index case, until August 13, 2022 (Table 1).
Materials and Methods
- The staff, patient, and visitor records were reviewed, and a total of 76 contacts were identified, comprising 44 infants, 29 HCWs or administrative staff, and 3 family members; of these, 75 contacts, except for an accountant who had been working from home, were identified as having potential exposure to the index case and were included in this investigation.
- The median (interquartile range) chronological and corrected ages of neonates at the start of the investigation were 30.0 days (20.8–38.3 days) and 19.5 days (7.5–32.3 days), respectively. Thirty-one neonates had received the BCG vaccination, while 13 had not. None had abnormal findings suggesting active TB on their CXRs. During TST screening at 3-months of age, 5 neonates, including 4 males and 1 female, tested positive and continued to receive LTBI treatment with 3-month rifampicin and isoniazid or 9-month isoniazid at the physician’s discretion. There was no significant difference between intradermal and subcutaneous BCG vaccination in regard to the induration diameters. Notably all neonates with positive TSTs had received the BCG vaccine (Table 2), resulting in a TST positivity rate of 16.1% (5/31 neonates) among the vaccinated. Positive TST findings were not associated with the duration of exposure to the index case (median 5.0 days among the 5 TST-positive neonates vs. 7.0 days among the 39 TST-negative neonates). In a year after exposure, no neonates, including the 5 with a positive TST, developed TB disease.
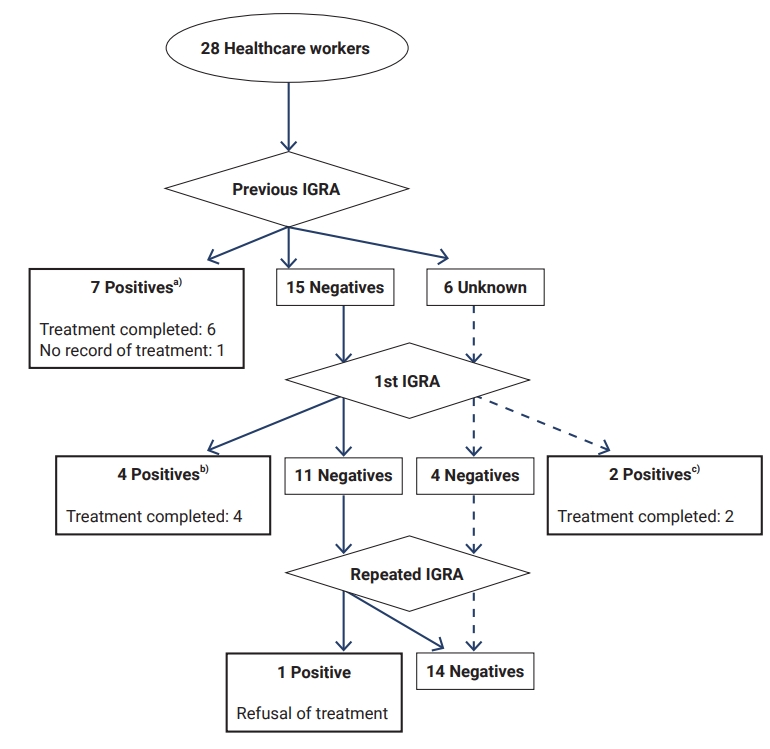
- The mean age of the 28 HCWs was 57.0±5.0 years, and 78.6% of HCWs were nursing staff. Twenty-two had previous IGRA results from 2015 to 2019, and 6 had no results recorded in the KNTSS. None of the 28 HCWs had abnormalities on the baseline or second CXRs. Six of the 21 HCWs, including 4 with negative results and 2 with no records from previous IGRAs, were positive on the first IGRA. One of the 15 HCWs who were negative at the first IGRA tested positive during repeated IGRA. At the physician’s discretion, 6 of the 7 HCWs with LTBI were treated with 4-month rifampin or 3-month rifampin and isoniazid (Figure 1). None of the 3 family members had any abnormalities suggestive of TB on either CXR. They tested negative on the first IGRAs, but 1 tested positive on repeated IGRA and received a 4-month rifampin regimen. During the study period, there were no cases of TB illness among the exposed HCWs.
Results
- Our study revealed that after an exposure investigation using TSTs/IGRAs, 13 contacts, including 5 neonates, 7 HCWs, and 1 household contact, were presumed to have LTBI with 12 contacts completing treatment for LTBI. Of the 44 neonates exposed to the index case, 5 (11.4%) had positive TST results at 3 months, significantly lower than the average 21.5% skin test-positive rate with 2 TU of PPD RT23 using a 10-mm cutoff point among those who had previous BCG vaccination without a TB exposure history [4]. None of the infants who had not received a BCG vaccination showed positive TST results. The estimated duration of exposure to the index case was not associated with positive TST results, and the induration diameters observed in neonates with positive results were barely over the cutoff value of 10 mm (range, 10–12 mm). Taken together, the risk of nosocomial TB transmission to newborns was estimated to be modest in this study, which is in line with previous research conducted in Canada, Australia, the United States, and South Korea [5–8].
- The index case had a minimal risk of transmission, as predicted by the negative sputum smear results and a lack of respiratory symptoms and cavities. It is noteworthy that LTBI screening and annual TB examinations for HCWs in postpartum care centers under South Korea’s amended Tuberculosis Prevention Act played a critical role in early detection and control of onward TB transmission [9]. Furthermore, the enhanced infection prevention and control measures implemented in the facility in response to COVID-19, such as physical distancing, wearing surgical masks or other types of masks with greater filtration efficiency, adherence to respiratory hygiene and cough etiquette, environmental disinfection, and mechanical ventilation, may have also substantially contributed to reducing the transmission of M. tuberculosis. In fact, a recent systematic review [10] found that the use of surgical masks in conjunction with cough etiquette training reduced TB infection by 14.8%, and mechanical ventilation was related to 2.9% to 14.8% less infection. Additionally, HCWs’ use of personal respirators reduced infection by 0% to 14.8%, all suggesting that infection control measures in healthcare settings are likely to reduce TB transmission.
- Six of the 28 HCWs (21.4%) exposed to the index case had positive results on the initial IGRA, and 1 (3.6%) had a positive conversion at 8 weeks on a repeated IGRA test. Although the prevalence of LTBI among HCWs in this study was substantially high, implying that the intensity of exposure to HCWs was high compared with that of neonates, the result should be interpreted with caution. The possibility that some HCWs with positive IGRA results could have been infected before the current exposure should be considered, given that the positivity rate of IGRA among HCWs was 24.1% overall and, notably, around 50% in HCWs in their 50s in another study [11], which is comparable to the average age of 57.0 years among the 7 LTBI-positive HCWs in our study. Nonetheless, our finding that the transmission rate among exposed HCWs was higher than that among exposed neonates is consistent with previous studies [5–8]. It is likely that neonates are better protected against exposure to airborne pathogens than HCWs and that neonates were cared for in baby cribs with minimal exposure with the index case.
- This study has several limitations. Indurations were measured by multiple individuals at 3 referral hospitals, which might have led to inter-observer variation. However, because they were all experienced healthcare personnel in TB management under the supervision of infectious disease specialists, this variation is expected to be modest. Second, the exposed neonates, HCWs, and household contacts could not be tracked for an extended period to ascertain whether they had developed TB. Finally, caution is warranted when extrapolating the findings of our study’s risk estimate of TB transmission to different healthcare settings since the transmission risk can vary depending on the intensity and duration of exposure, the infectiousness of an index case, and environmental factors.
- In summary, although 5 neonates were treated with prophylactic TB medication for fear of severe outcomes, this study demonstrated that transmission to neonates exposed to active pulmonary TB at a postpartum care center was minimal, especially under enhanced infection prevention and control measures. Our findings highlight the importance of early detection of subclinical TB through annual TB examinations, which is conducive to reducing the intensity and duration of TB exposure in healthcare settings in a country with an intermediate TB burden.
Discussion
-
Ethics Approval
The requirement for written informed consent from participants was waived according to the Korean Infectious Disease Control and Prevention Act (No. 4). The present study protocol was reviewed and approved by the Institutional Review Board of the Korea Diseases Control and Prevention Agency (2022-04-08-PE-A).
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: IKK, YAK, JSS; Data curation: IKK, SJK, KHB; Data interpretation: IKK, YAK, JSS; Investigation: SJK, KHB, JEO, MGL, MYK, JSS; Supervision: MYK, JSS; Writing-original draft: IKK; Writing-review & editing: all authors.
-
Additional Contributions
We thank the public health officers at the Suji-gu Public Health Center and the medical professionals at the referral hospitals for their contributions to the investigations and patient management.
Article information

| Characteristic | Neonate (n=44) |
|---|---|
| Age at start of the investigation (d) | |
| Chronologicala) | 30.0 (20.8–38.3) |
| Correctedb) | 19.5 (7.5–32.3) |
| Gestational age (wk) | 38.5 (38.1–39.3) |
| Sex | |
| Male | 22 (50.0) |
| Female | 22 (50.0) |
| BCG vaccination | |
| Vaccinated | 31 (70.5) |
| Intradermal | 19 (61.3) |
| Subcutaneous | 12 (38.7) |
| Unvaccinated | 13 (29.5) |
| TST | |
| Positive | 5 (11.4)c) |
| Negative | 39 (88.6) |
Data are presented as median (interquartile range) or n (%).
TST, tuberculin skin test; BCG, Bacille Calmette-Guérin.
a) Chronological age refers to the number of weeks since the date of birth.
b) Corrected age refers to the number of weeks since the expected due date.
c) Two of the 5 infants had an induration diameter of 10 mm and 3 had induration diameters of 12 mm. All 5 had been BCG-vaccinated: 2 with intradermal injections and 3 with subcutaneous injections. Three of them were treated with 3 months of rifampicin and isoniazid and 2 infants with 9 months of isoniazid.
- 1. Marais BJ, Gie RP, Schaaf HS, et al. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med 2006;173:1078−90.ArticlePubMed
- 2. Ministry of Health and Welfare (MOHW). The status of sanhujoriwon in South Korea [Internet]. Sejong: MOHW; 2022 [cited 2022 Aug 17]. Available from: http://www.mohw.go.kr/react/jb/sjb030301vw.jsp?PAR_MENU_ID=03&MENU_ID=0321&CONT_SEQ=373123. Korean.
- 3. Korea Disease Control and Prevention Agency (KDCA). Guidelines on tuberculosis management [Internet]. Chungju: KDCA; 2022 [cited 2022 Aug 17]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019#. Korean.
- 4. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax 2002;57:804−9.PubMedPMC
- 5. Sen M, Gregson D, Lewis J. Neonatal exposure to active pulmonary tuberculosis in a health care professional. CMAJ 2005;172:1453−6.ArticlePubMedPMC
- 6. Nania JJ, Skinner J, Wilkerson K, et al. Exposure to pulmonary tuberculosis in a neonatal intensive care unit: unique aspects of contact investigation and management of hospitalized neonates. Infect Control Hosp Epidemiol 2007;28:661−5.ArticlePubMed
- 7. Ahn JG, Kim DS, Kim KH. Nosocomial exposure to active pulmonary tuberculosis in a neonatal intensive care unit. Am J Infect Control 2015;43:1292−5.ArticlePubMed
- 8. Fisher KE, Guaran R, Stack J, et al. Nosocomial pulmonary tuberculosis contact investigation in a neonatal intensive care unit. Infect Control Hosp Epidemiol 2013;34:754−6.ArticlePubMed
- 9. Ministry of Government Legislation (MOGL). Article 11 (tuberculosis examination, etc.) of Tuberculosis Prevention Act in South Korea [Internet]. Sejong: MOGL; 2022 [cited 2022 Aug 17]. Available from: https://www.law.go.kr/LSW/eng/engLsSc.do?menuId=2§ion=lawNm&query=Tuberculosis+prevention&x=0&y=0#EJ11:0. Korean.
- 10. Fox GJ, Redwood L, Chang V, et al. The effectiveness of individual and environmental infection control measures in reducing the transmission of Mycobacterium tuberculosis: a systematic review. Clin Infect Dis 2021;72:15−26.ArticlePubMedPDF
- 11. Lee EH, Son NH, Kwak SH, et al. Decreased annual risk of tuberculosis infection in South Korean healthcare workers using interferon-gamma release assay between 1986 and 2005. BMC Infect Dis 2021;21:1161. ArticlePubMedPMCPDF
References
Figure & Data
References
Citations



 Cite
Cite