Previous issues
- Page Path
- HOME > Articles and issues > Previous issues
Editorial
- Efforts to return to a normal society
- Jong-Koo Lee
- Osong Public Health Res Perspect. 2022;13(6):391-393. Published online December 30, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.13.6.01
- 1,088 View
- 54 Download
Review Article
- SARS-CoV-2 in brief: from virus to prevention
- Hassan Karami, Zeinab Karimi, Negin Karami
- Osong Public Health Res Perspect. 2022;13(6):394-406. Published online November 28, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0155
- 2,783 View
- 94 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - The recent outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), ahighly transmissible virus with a likely animal origin, has posed major and unprecedentedchallenges to millions of lives across the affected nations of the world. This outbreak firstoccurred in China, and despite massive regional and global attempts shortly thereafter, itspread to other countries and caused millions of deaths worldwide. This review presents keyinformation about the characteristics of SARS-CoV-2 and its associated disease (namely,coronavirus disease 2019) and briefly discusses the origin of the virus. Herein, we also brieflysummarize the strategies used against viral spread and transmission.
-
Citations
Citations to this article as recorded by- Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic
Radu Lefter, Prairna Balyan, Ioana-Miruna Balmus, Abdellah Ech-Chahad, Ahmad Ali, Alin Ciobica, Antoneta Dacia Petroaie, Gabriela Halitchi, Bogdan Novac, Catalina Ionescu, Fatima Zahra Kamal
Microbiology Research.2024; 15(2): 525. CrossRef - Surveillance of endemic coronaviruses during the COVID‐19 pandemic in Iran, 2021–2022
Hassan Karami, Kaveh Sadeghi, Sevrin Zadheidar, Fatemeh Saadatmand, Negar Mirsalehi, Nima Hoveidi Ardestani, Shirin Kalantari, Mohammad Farahmand, Jila Yavarian, Talat Mokhtari‐Azad
Influenza and Other Respiratory Viruses.2023;[Epub] CrossRef
- Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic
Review Article
- Carbapenem resistance in critically important human pathogens isolated from companion animals: a systematic literature review
- Angie Alexandra Rincón-Real, Martha Cecilia Suárez-Alfonso
- Osong Public Health Res Perspect. 2022;13(6):407-423. Published online December 16, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0033
- 3,004 View
- 149 Download
- 4 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - This study aimed to describe the presence and geographical distribution of Gram-negativebacteria considered critical on the priority list of antibiotic-resistant pathogens publishedby the World Health Organization, including carbapenem-resistant Enterobacteriaceae,carbapenem-resistant Acinetobacter spp., and carbapenem-resistant Pseudomonas aeruginosa.A systematic review of original studies published in 5 databases between 2010 and 2021 wasconducted, including genotypically confirmed carbapenem-resistant isolates obtained fromcanines, felines, and their settings. Fifty-one articles met the search criteria. Carbapenemresistant isolates were found in domestic canines and felines, pet food, and on veterinarymedical and household surfaces. The review found that the so-called “big five”—that is, the5 major carbapenemases identified worldwide in Enterobacterales (New Delhi metallo-βlactamase, active-on-imipenem, Verona integron-encoded metallo-β-lactamase, Klebsiellapneumoniae carbapenemase, and oxacillin [OXA]-48-like)—and the 3 most importantcarbapenemases from Acinetobacter spp. (OXA-23-like, OXA-40-like, and OXA-58-like) hadbeen detected in 8 species in the Enterobacteriaceae family and 5 species of glucose nonfermenting bacilli on 5 continents. Two publications used molecular analysis to confirmcarbapenem-resistant bacteria transmission between owners and dogs. Isolating criticallyimportant human carbapenem-resistant Gram-negative bacteria from domestic canines andfelines highlights the importance of including these animal species in surveillance programsand antimicrobial resistance containment plans as part of the One Health approach.
-
Citations
Citations to this article as recorded by- First report of a blaNDM-5-carrying Escherichia coli sequence type 12 isolated from a dog with pyometra in Japan
Kazuki Harada, Tadashi Miyamoto, Michiyo Sugiyama, Tetsuo Asai
Journal of Infection and Chemotherapy.2024;[Epub] CrossRef - The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022
EFSA Journal.2024;[Epub] CrossRef - Epidemiological analysis and prevention strategies in response to a shigellosis cluster outbreak: a retrospective case series in an alternative school in the Republic of Korea, 2023
Yeongseo Ahn, Sunmi Jin, Gemma Park, Hye Young Lee, Hyungyong Lee, Eunkyung Shin, Junyoung Kim, Jaeil Yoo, Yuna Kim
Osong Public Health and Research Perspectives.2024; 15(1): 68. CrossRef - The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021
EFSA Journal.2023;[Epub] CrossRef - Resistome-based surveillance identifies ESKAPE pathogens as the predominant gram-negative organisms circulating in veterinary hospitals
Flavia Zendri, Cajsa M. Isgren, Jane Devaney, Vanessa Schmidt, Rachel Rankin, Dorina Timofte
Frontiers in Microbiology.2023;[Epub] CrossRef - Unveiling the emergence of multidrug-resistant pathogens in exotic pets from France: a comprehensive study (2017-2019)
Sandro Cardoso, Aurélie Le Loc’h, Inês Marques, Anabela Almeida, Sérgio Sousa, Maria José Saavedra, Sofia Anastácio, Eduarda Silveira
One Health & Implementation Research.2023; 3(4): 161. CrossRef
- First report of a blaNDM-5-carrying Escherichia coli sequence type 12 isolated from a dog with pyometra in Japan
Original Articles
- Time-series comparison of COVID-19 case fatality rates across 21 countries with adjustment for multiple covariates
- Yongmoon Kim, Bryan Inho Kim, Sangwoo Tak
- Osong Public Health Res Perspect. 2022;13(6):424-434. Published online November 28, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0212
- 2,694 View
- 111 Download
- 1 Web of Science
- 1 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Objectives
Although it is widely used as a measure for mortality, the case fatality rate (CFR) ofcoronavirus disease 2019 (COVID-19) can vary over time and fluctuate for many reasons otherthan viral characteristics. To compare the CFRs of different countries in equal measure, weestimated comparable CFRs after adjusting for multiple covariates and examined the mainfactors that contributed to variability in the CFRs among 21 countries.Methods: For statistical analysis, time-series cross-sectional data were collected from OurWorld in Data, CoVariants.org, and GISAID. Biweekly CFRs of COVID-19 were estimated bypooled generalized linear squares regression models for the panel data. Covariates includedthe predominant virus variant, reproduction rate, vaccination, national economic status,hospital beds, diabetes prevalence, and population share of individuals older than age 65. Intotal, 21 countries were eligible for analysis.Results: Adjustment for covariates reduced variation in the CFRs of COVID-19 across countriesand over time. Regression results showed that the dominant spread of the Omicron variant,reproduction rate, and vaccination were associated with lower country-level CFRs, whereasage, the extreme poverty rate, and diabetes prevalence were associated with higher countrylevel CFRs.Conclusion: A direct comparison of crude CFRs among countries may be fallacious, especiallyin a cross-sectional analysis. Our study presents an adjusted comparison of CFRs over timefor a more proper comparison. In addition, our findings suggest that comparing CFRs amongdifferent countries without considering their context, such as the epidemic phase, medicalcapacity, surveillance strategy, and socio-demographic traits, should be avoided. -
Citations
Citations to this article as recorded by- Comments on the article "Time-series comparison of COVID-19 case fatality rates across 21 countries with adjustment for multiple covariates"
Gaetano Perone
Osong Public Health and Research Perspectives.2023; 14(2): 146. CrossRef
- Comments on the article "Time-series comparison of COVID-19 case fatality rates across 21 countries with adjustment for multiple covariates"
- mRNA vaccine effectiveness against SARS-CoV-2 B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant transmission from home care cases to household contacts in South Korea
- Hanul Park, Young Joon Park, Sang Eun Lee, Min Jei Lee, Hyungtae Ahn
- Osong Public Health Res Perspect. 2022;13(6):435-442. Published online November 28, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0243
- 4,711 View
- 171 Download
- 1 Web of Science
- 1 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 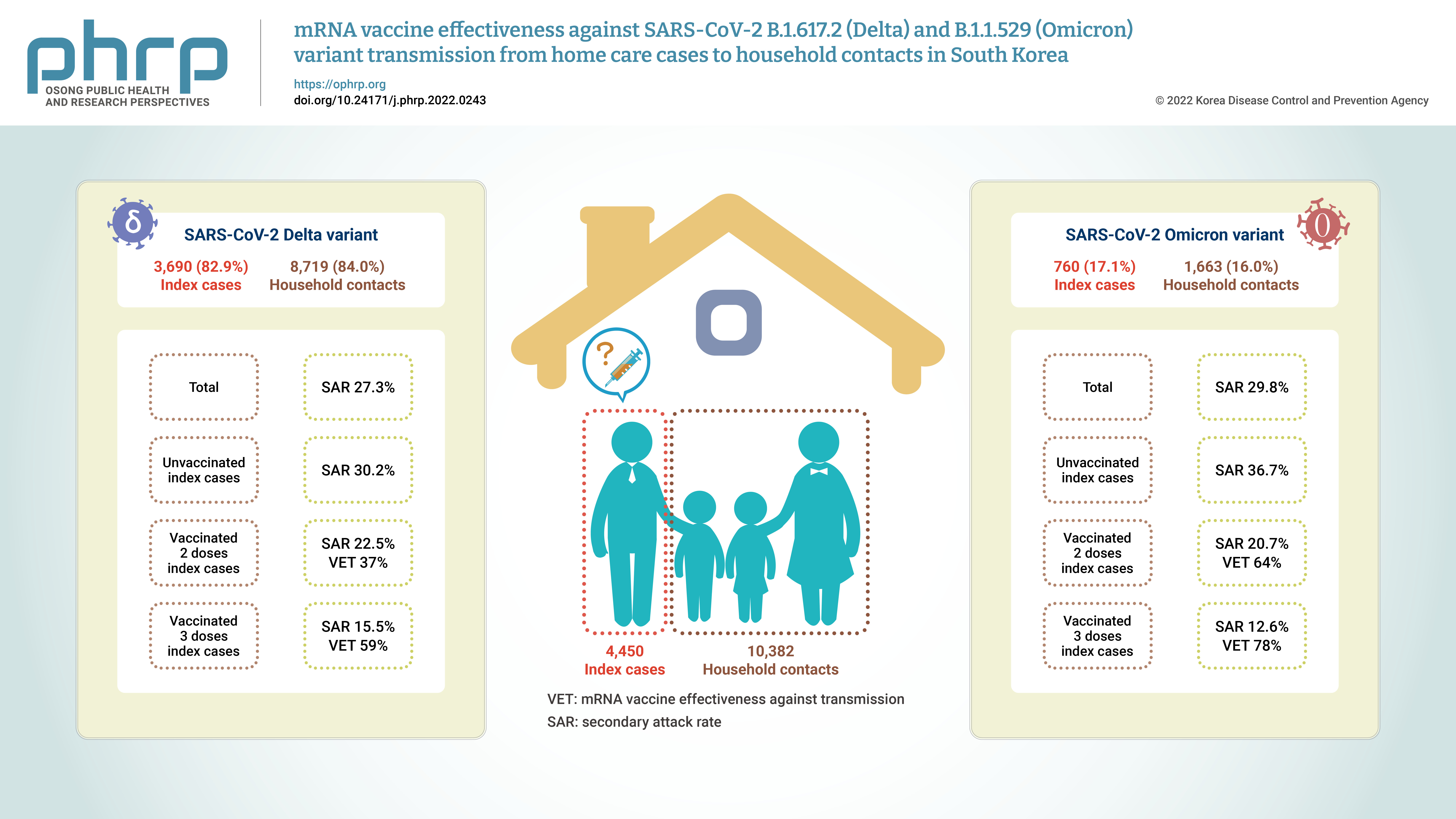
- Objectives
Household contacts of confirmed cases of coronavirus disease 2019 (COVID-19) areexposed to a high risk of viral transmission, and secondary incidence is an important indicatorof community transmission. This study analyzed the secondary attack rate and mRNA vaccineeffectiveness against transmission (VET) for index cases (patients treated at home) confirmedto be infected with the Delta and Omicron variants.Methods: The subjects of the study were 4,450 index cases and 10,382 household contacts.Logistic regression analysis was performed to compare the secondary attack rate byvaccination status, and adjusted relative risk and 95% confidence intervals were identified.Results: The secondary attack rate of the Delta variant was 27.3%, while the secondary attackrate of the Omicron variant was 29.8%. For the Delta variant, groups with less than 90 daysand more than 90 days after 2 doses of mRNA vaccination both showed a VET of 37%. For theOmicron variant, a 64% VET was found among those with less than 90 days after 2 doses ofmRNA vaccination.Conclusion: This study provides useful data on the secondary attack rate and VET of mRNAvaccines for household contacts of COVID-19 cases in South Korea. -
Citations
Citations to this article as recorded by- Household secondary attack rates and risk factors during periods of SARS-CoV-2 Delta and Omicron variant predominance in the Republic of Korea
Jin Lee, Mijeong Ko, Seontae Kim, Dosang Lim, Gemma Park, Sang-Eun Lee
Osong Public Health and Research Perspectives.2023; 14(4): 263. CrossRef
- Household secondary attack rates and risk factors during periods of SARS-CoV-2 Delta and Omicron variant predominance in the Republic of Korea
Brief Reports
- The effectiveness of Paxlovid treatment in long-term care facilities in South Korea during the outbreak of the Omicron variant of SARS-CoV-2
- Hanul Park, Young Joon Park, Hye Young Lee, Mi Yu, Yeong-Jun Song, Sang Eun Lee, Ji-Joo Lee, Eun-Sol Lee, Yeonjung Kim
- Osong Public Health Res Perspect. 2022;13(6):443-447. Published online December 23, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0262
- 3,109 View
- 214 Download
- 7 Web of Science
- 9 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 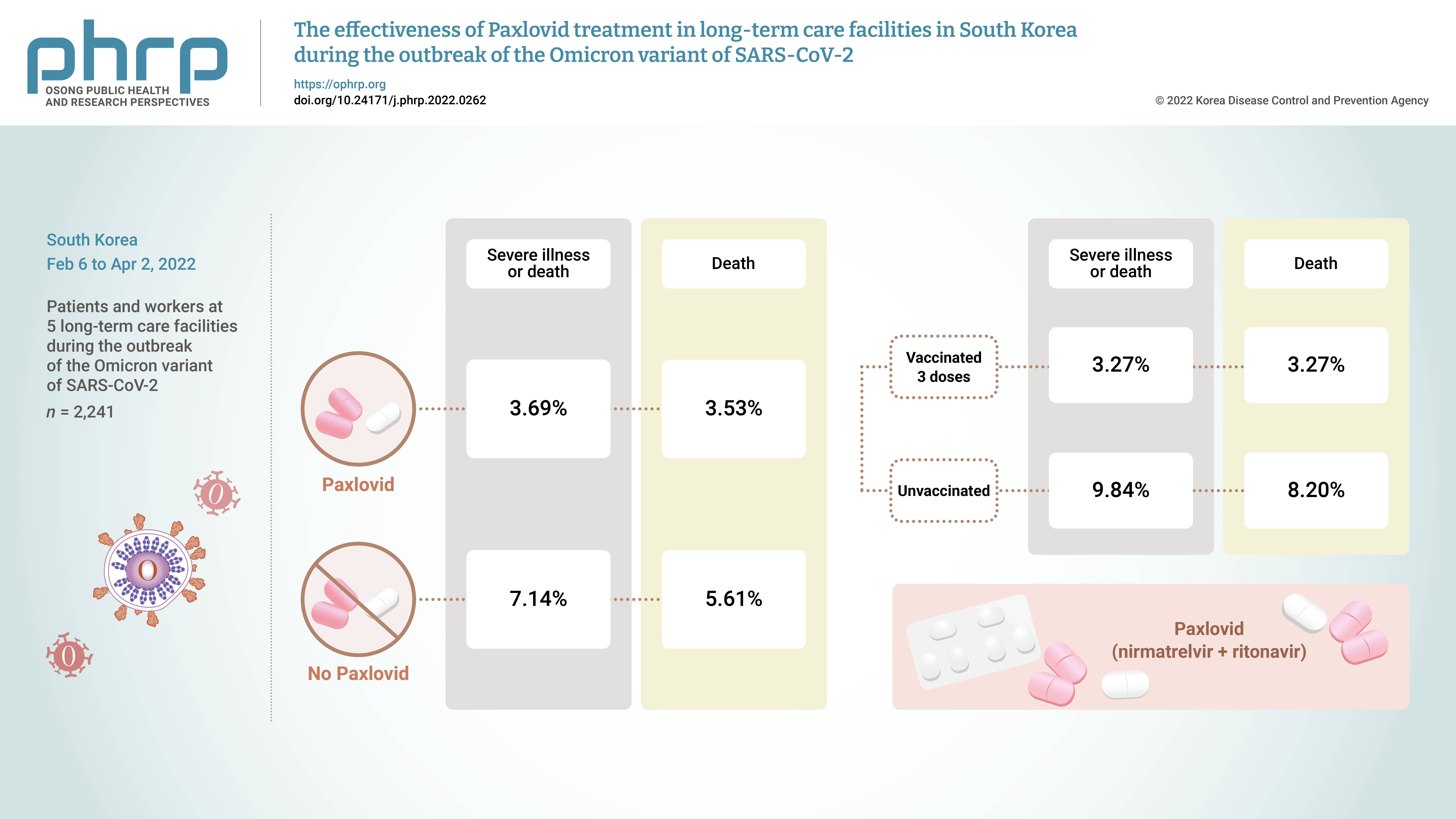
- Objectives
On November 5, 2021, Pfizer Inc. announced Paxlovid (nirmatrelvir +ritonavir) asa treatment method that could reduce the risk of hospitalization or death for patients withconfirmed coronavirus disease 2019 (COVID-19).Methods: From February 6, 2022 to April 2, 2022, the incidence of COVID-19 and the effectsof treatment with Paxlovid were analyzed in 2,241 patients and workers at 5 long-term carefacilities during the outbreak of the Omicron variant of severe acute respiratory syndromecoronavirus 2 in South Korea.Results: The rate of severe illness or death in the group given Paxlovid was 51% lower thanthat of the non-Paxlovid group (adjusted risk ratio [aRR], 0.49; 95% confidence interval [CI],0.24−0.98). Compared to unvaccinated patients, patients who had completed 3 doses of thevaccine had a 71% reduced rate of severe illness or death (aRR, 0.29; 95% CI, 0.13−0.64) and a65% reduced death rate (aRR, 0.35; 95% CI, 0.15−0.79).Conclusion: Patients given Paxlovid showed a lower rate of severe illness or death and alower fatality rate than those who did not receive Paxlovid. Patients who received 3 dosesof the vaccine had a lower rate of severe illness or death and a lower fatality rate than theunvaccinated group. -
Citations
Citations to this article as recorded by- Efficacy and safety of antiviral treatments for symptomatic COVID-19 outpatients: Systematic review and network meta-analysis
Meital Zur, Thalia Peselev, Stav Yanko, Victoria Rotshild, Ilan Matok
Antiviral Research.2024; 221: 105768. CrossRef - Clinical Effectiveness of Ritonavir-Boosted Nirmatrelvir—A Literature Review
Sydney Paltra, Tim O. F. Conrad
Advances in Respiratory Medicine.2024; 92(1): 66. CrossRef - Effectiveness of nirmatrelvir‐ritonavir on severe outcomes of COVID‐19 in the era of vaccination and Omicron: An updated meta‐analysis
Sien Ombelet, Diego Castanares‐Zapatero, Fabian Desimpel, Frank Hulstaert, Sabine Stordeur, Dominique Roberfroid
Journal of Medical Virology.2024;[Epub] CrossRef - COVID‐19 infection in patients with haematological malignancies: A single‐centre survey in the latest Omicron wave in China
Xiaolu Zhu, Qian Jiang, Jin Lu, Yuqian Sun, Xiaosu Zhao, Shenmiao Yang, Feifei Tang, Wenjing Yu, Ting Zhao, Xiaohong Liu, Jinsong Jia, Wenbing Duan, Lijuan Hu, Jing Wang, Yang Liu, Nan Peng, Xuelin Dou, Rui Ma, Qiang Fu, Huifang Wang, Kaiyan Liu, Xiaojun
British Journal of Haematology.2023; 202(1): 31. CrossRef - The association mental health of adolescents with economic impact during the COVID-19 pandemic: a 2020 Korean nationally representative survey
Hanul Park, Kang-Sook Lee
BMC Public Health.2023;[Epub] CrossRef - Efficacy and safety of paxlovid (nirmatrelvir/ritonavir) in the treatment of COVID‐19: An updated meta‐analysis and trial sequential analysis
Haokun Tian, Changsen Yang, Tiangang Song, Kechen Zhou, Lequan Wen, Ye Tian, Lirui Tang, Weikai Xu, Xinyuan Zhang
Reviews in Medical Virology.2023;[Epub] CrossRef - Real-World Effectiveness of Nirmatrelvir-Ritonavir and Its Acceptability in High-Risk COVID-19 Patients
Min-Kyung Kim, Kyung-Shin Lee, Sin Young Ham, Youn Young Choi, Eunyoung Lee, Seungjae Lee, Bora Lee, Jaehyun Jeon, BumSik Chin, Yeonjae Kim, Gayeon Kim, Hee-Chang Jang, Jae-Phil Choi, Sang-Won Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score Matched Study
Hye Rim Park, Min-Gyu Yoo, Jong Mu Kim, Soon Jong Bae, Hyungmin Lee, Jungyeon Kim
Infection & Chemotherapy.2023; 55(4): 490. CrossRef - Nirmatrelvir combined with ritonavir for preventing and treating COVID-19
Stefanie Reis, Maria-Inti Metzendorf, Rebecca Kuehn, Maria Popp, Ildiko Gagyor, Peter Kranke, Patrick Meybohm, Nicole Skoetz, Stephanie Weibel
Cochrane Database of Systematic Reviews.2023;[Epub] CrossRef
- Efficacy and safety of antiviral treatments for symptomatic COVID-19 outpatients: Systematic review and network meta-analysis
- A low risk of nosocomial transmission of subclinical tuberculosis to neonates in a postpartum care center under COVID-19 control measures
- In Kyoung Kim, So Jung Kim, Kyoung Hee Bae, Mi Young Kim, Ji Eun Oh, Mi Gyeong Lee, Young Ae Kang, Jin Su Song
- Osong Public Health Res Perspect. 2022;13(6):448-452. Published online December 16, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0235
- 2,087 View
- 98 Download
-
 Abstract
Abstract
 PDF
PDF - We report the results of investigating and managing a tuberculosis (TB) exposure in apostpartum care center. Among the contacts exposed to a nursing assistant with subclinical TB,5 of 44 neonates (11.4%) had positive tuberculin skin tests (TSTs) at 3 months of age, and all theTST-positive neonates received the Bacille Calmette-Guérin vaccination. Seven of 28 healthcareworkers (25.0%) and 1 of 3 household contacts (33.3%) were positive in the initial or repeatedinterferon-gamma release assay. None of the contacts developed TB disease during the studyperiod. Annual TB examinations of healthcare personnel at a postpartum care center under theTuberculosis Prevention Act in South Korea enabled the early detection of subclinical TB, whichreduced the risk of transmission to neonates under strict coronavirus disease 2019 preventionmeasures.





 First
First Prev
Prev


