Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 12(2); 2021 > Article
-
Original Article
Seroprevalence of antibodies to SARS-CoV-2 and predictors of seropositivity among employees of a teaching hospital in New Delhi, India -
Pragya Sharma1
 , Rohit Chawla2
, Rohit Chawla2 , Ritika Bakshi1
, Ritika Bakshi1 , Sonal Saxena2
, Sonal Saxena2 , Saurav Basu1
, Saurav Basu1 , Pradeep Kumar Bharti2
, Pradeep Kumar Bharti2 , Meera Dhuria3
, Meera Dhuria3 , S. K. Singh3
, S. K. Singh3 , Panna Lal1
, Panna Lal1
-
Osong Public Health and Research Perspectives 2021;12(2):88-95.
DOI: https://doi.org/10.24171/j.phrp.2021.12.2.06
Published online: April 9, 2021
1Department of Community Medicine, Maulana Azad Medical College, New Delhi, India
2Department of Microbiology, Maulana Azad Medical College, New Delhi, India
3Division of Epidemiology, National Centre for Disease Control, Delhi, India
- Corresponding author: Saurav Basu Department of Community Medicine, Maulana Azad Medical College, 2 BSZ Marg, New Delhi 110002, India E-mail: saurav.basu1983@gmail.com
© 2021 Korea Disease Control and Prevention Agency
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- Healthcare workers (HCWs) are at a high risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to the increased likelihood of clinical exposure during patient management. The study objective was to determine the seroprevalence of antibodies to SARS-CoV-2 and its predictors among hospital employees.
-
Methods
- The cross-sectional study was conducted at a teaching hospital from August 2020 to September 2020 among 1,401 employees, including 1,217 HCWs, in New Delhi, India. The serum samples were examined for immunoglobulin G (IgG) antibodies to SARS-CoV-2 using the COVID Kavach-Anti-SARS-CoV-2 IgG Antibody Detection enzyme-linked immunosorbent assay kit. Data were collected electronically using the EpiCollect mobile platform. A p < 0.05 was considered to indicate statistical significance.
-
Results
- A total of 169 participants (12.1%) had detectable IgG antibodies to SARS-CoV-2. The highest seropositivity rate was observed in the administrative staff (20.1%), while it was lowest among medical doctors (5.5%, p < 0.001). Male sex and ever having lived in a containment zone were independently associated with past infection with SARS-CoV-2.
-
Conclusion
- The seroprevalence of SARS-CoV-2 infection in health workers may be lower than in the general population in New Delhi. However, nonpharmaceutical interventions were not associated with a reduction in the risk of acquisition of SARS-CoV-2.
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as a global pandemic since its initial identification in December 2019 [1]. The clinical spectrum of coronavirus disease 2019 (COVID-19), which is caused by SARS-CoV-2, ranges from asymptomatic to severe respiratory symptoms and death, with significantly higher mortality in individuals with comorbidities and among elderly patients [2]. In India, the second-most populous country of the world, 6.7 million cases and 104,555 deaths attributed to COVID-19 have been recorded as of October 7, 2020. In the state of New Delhi, the Indian capital city; 295,236 cases and 5,581 deaths were observed during the same period [3].
- It is well-established that healthcare workers (HCWs) comprise a population subgroup that is at a high risk of contracting SARS-CoV-2 infection due to clinical exposure during the management of suspected or confirmed COVID-19 patients [4]. The risk is further accentuated due to the incorrect use of personal protective equipment (PPE) and nonadherence to the recommended infection prevention and control guidelines [5].
- The World Health Organization has estimated that 1.4 million COVID-19 infections occurred among HCWs worldwide as of August 2020 [6]. In India, according to an unofficial estimate, a total of 2,174 and 382 COVID-19 related infections and deaths, respectively, have been reported among medical doctors as of September 10, 2020, highlighting a 16-fold higher case fatality rate compared to the general population [7].
- Early identification, isolation, and treatment of HCWs are indispensable tools for limiting the spread of SARS-CoV-2 among co-workers and non-COVID-19 patients within a healthcare facility. Understanding the risk factors related to the transmission of infection within healthcare settings can guide effective planning and implementation of evidence-based strategies for protecting HCWs.
- Establishing the true infection rate of SARS-CoV-2 among HCWs requires an assessment of antibody seroprevalence since asymptomatic cases constitute approximately 40% to 45% of all SARS-CoV-2 infections [8]. Therefore, we conducted this study to determine the seroprevalence of antibodies to SARS-CoV-2 and its predictors among employees of a teaching hospital in New Delhi, India.
Introduction
- Study Design and Setting
- We conducted a cross-sectional study from August 2020 to September 2020 at a teaching hospital located in New Delhi, India. The tertiary care hospital was designated as a dedicated COVID-19 hospital in April 2020, and has since treated more than 9,000 moderate to severe cases of COVID-19 [9]. No non-COVID-19 patients were admitted or treated at the hospital during the pandemic.
- HCWs at the hospital deployed to the COVID-19 isolation wards currently work 15-day shifts, followed by 7 days of quarantine and another 7 days of home isolation. All HCWs working at the hospital could opt to be tested for COVID-19 if they self-reported any breach in PPE or developing symptoms suggestive of the disease. Medical personnel attending to COVID-19 patients in the wards and intensive care units were provided complete PPE kits including gowns, gloves, and N95 respirators. Administrative staff who were unlikely to come in contact with suspected COVID-19 patients were provided with surgical masks. Wearing an N95 or a surgical mask was mandatory for all hospital employees. Furthermore, employees of the hospital without any preexisting cardiac abnormalities were recommended the to receive the Indian Council of Medical Research-approved hydroxychloroquine (HCQ) prophylaxis regimen, the intake of which was completely voluntary [10].
- Sampling Strategy
- Sites for immunoglobulin G (IgG) SARS-CoV-2 antibody testing were organized at different locations in the medical school and the hospital complex, and information about testing sites was disseminated through notice boards, banners, flyers, and WhatsApp groups. All the employees were invited to participate in the study. The hospital campus includes a medical college, a dental college, an eye hospital, and a tertiary care hospital with approximately 3,000 employees. Participation in this study was a one-time activity, and seronegative participants were not retested subsequently.
- Inclusion Criteria
- We included doctors, nurses, housekeeping staff, biomedical waste collectors, and administrative staff in the study.
- Methodology
- The participants were informed that seropositivity would indicate past infection. A blood sample (3 mL) was collected in a serum separator tube under aseptic conditions and transported to the testing facility upright in a transport box within 3 hours after collection. The serum samples were tested for qualitative detection of IgG antibodies to SARS-CoV-2 using the COVID Kavach-Anti-SARS-CoV-2 Human IgG ELISA Kit (ICMR-NIV, Pune, India). The sensitivity and specificity of the assay have been reported to be 92.4% and 97.9%, respectively [11].
- Data Collection
- We collected participant information on sociodemographic variables and on occupational exposure-related risk factors (Table S1) through face-to-face interviews, and the data were captured using the paperless mobile EpiCollect platform [12].
- Operational Definitions
- Participants were characterized as high-risk if they were directly involved in the care of COVID-19 patients or handling of their clinical samples, and were considered low-risk otherwise. Containment zones were defined by the local administration, usually as places within a 1-km radius where 3 or more cases were detected. Within these zones, movement and access to the neighborhood were significantly restricted with exemptions for essential services staff [13].
- Statistical Analysis
- Data were analyzed with IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). The results were expressed as frequency and proportions for categorical variables, and mean and standard deviation for continuous variables. Associations between categorical variables were assessed using the chi-square test. The variables that were significantly associated with the presence of the IgG antibody to SARS-CoV-2 were included in a binary logistic regression model. A p < 0.05 was considered to statistical significance.
Materials and Methods
- Sociodemographic Characteristics
- We enrolled a total of 1,401 participants, including 769 males (54.9%) and 632 females (45.1%). The median age of the participants was 33 years (interquartile range, 27−45 years). The participants comprised doctors (43.8%), nursing personnel (18.1%), auxiliary health workers (25.1%), and administrative staff (13.1%). A total of 466 participants (33.3%) reported engaging in high-risk activities.
- Seroprevalence of IgG Antibodies to SARS-CoV-2
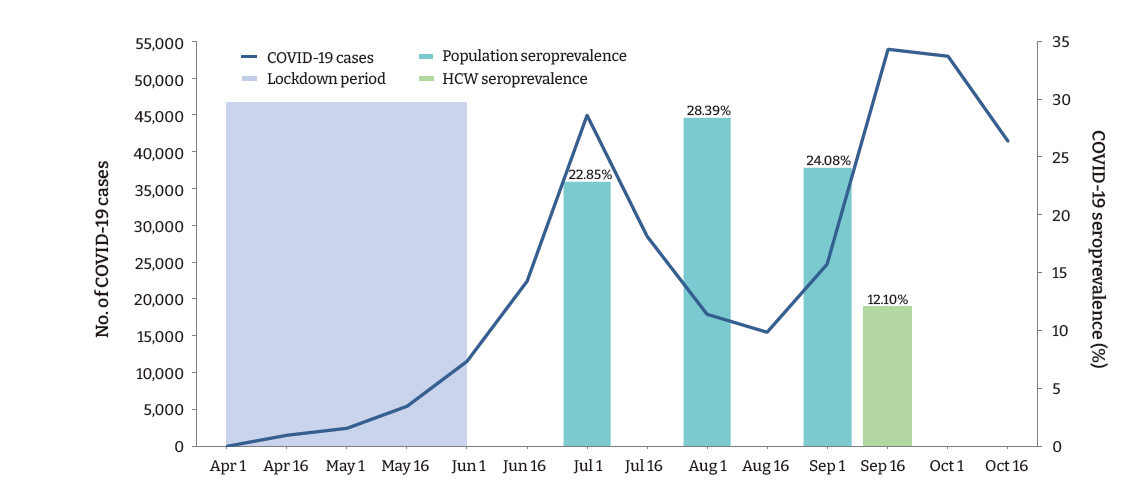
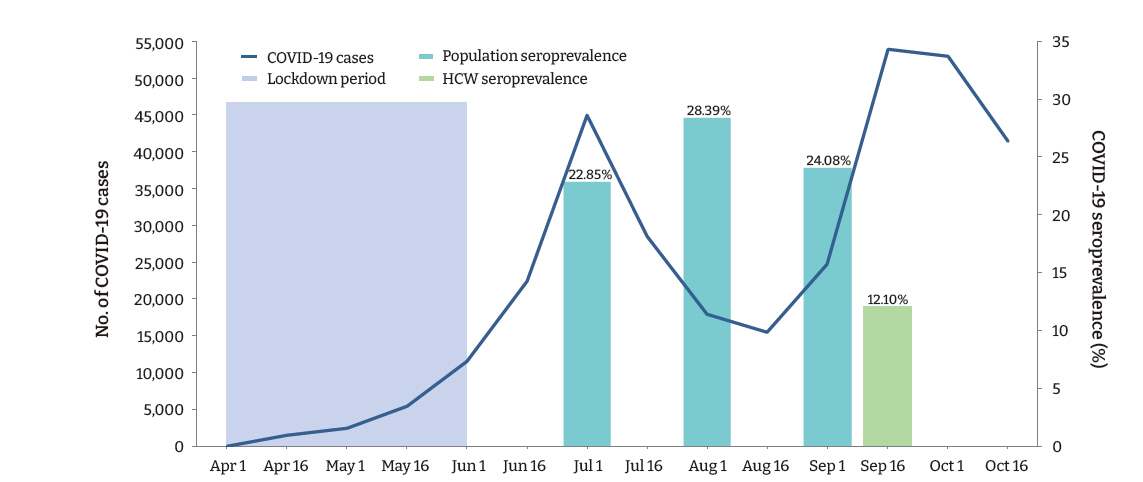
- A total of 169 (12.1%; 95% confidence interval [CI], 10.4−13.8) participants, and 132 (10.8%; 95% CI, 9.1−12.6) (n=1,217) HCWs (doctors and nurses), had detectable IgG antibodies against SARS-CoV-2. Among the various categories of participants, administrative staff (20.1%) had the highest seropositivity rate, followed by auxiliary staff (18.5%), nursing staff (13.0%), and doctors (5.5%; p < 0.001). The seroprevalence of SARS-CoV-2 among the healthcare employees observed in this study was less than half of that observed in the general population in the state during a similar period (Figure 1).
- In the bivariate analysis, the seropositivity was significantly higher in participants aged ≥34 years, of the male sex, performing administrative duties, having low body mass index (<18.5 kg/m2), with a history of COVID-19 diagnosis through real-time polymerase chain reaction (PCR) or rapid antigen test, and a history of having ever consumed any alternative medication (Table 1). Although not statistically significant, the seropositivity was higher in participants with any self-reported preexisting chronic illness (p=0.062) and in those who did not undertake the HCQ prophylaxis regimen (p=0.246).
- Among the 101 participants with a previous history of COVID-19 infection diagnosed with real-time PCR or rapid antigen test, 42 participants (41.6%) were IgG-seropositive. In the participants with an absence of documented COVID-19 infection (n=1,300), 127 (9.76%) were IgG-seropositive. Moreover, the proportion of health workers with seropositivity was higher in those who reported a breach of PPE during patient management compared to those who did not, but this difference did not reach statistical significance (p=0.139) (Table 2).
- We conducted a binary logistic regression analysis by including variables that were statistically significant in the bivariate analysis (p < 0.05). The model was found to be statistically significant (χ2 (4)=69.76; p < 0.001). The model correctly classified 87.9% of cases. The Hosmer-Lemeshow goodness of fit test-statistic had a p-value of 0.415, from which we concluded that the model estimated the data acceptably. Among the variables that were found to be statistically significant in the bivariate analysis, only male sex and the type of occupation were found to be independently associated with seropositivity in the logistic regression analysis (Table 3). The administrative staff had 3.8-fold higher odds of being IgG-seropositive than the doctors.
Results
- The present study conducted among employees of a teaching hospital in New Delhi observed that the IgG antibody to SARS-CoV-2 was present in 12.1% of participants, indicating past infection with the virus. A large community-based repeated serosurvey conducted in Delhi during the same period reported detectable IgG antibodies in 24.08% to 28.39% of the population [14]. Surprisingly, in our study, the seropositivity was significantly higher among administrative staff who had a considerably lower risk of exposure to the virus than HCWs. These findings suggest that the likelihood of exposure to SARS-CoV-2 was high in the community. Furthermore, although the administrative staff were provided with face-masks and instructed to adhere to hand hygiene and social distancing measures, a lack of effective training in correctly using PPE may have disproportionately accentuated their risk compared to the HCWs.
- Previous studies in India have shown variable rates of seropositivity among HCWs ranging from 2.5% in the Srinagar district [15] to 16% in Southern Rajasthan, with most cases being asymptomatic [16]. In Europe, various Italian studies have reported COVID-19 IgG seroprevalence among hospital personnel to range from 5.1% to 7.4% [17,18]. In Denmark, the seropositivity among HCWs was 4.04%, with a significant association observed with a history of exposure to COVID-19 patients [19]. Another study in the USA reported 6% of HCWs within a multistate hospital network had detectable SARS-CoV-2 antibodies, and within the seropositive subgroup, 69% had no prior microbiological confirmation of COVID-19 disease [20]. However, a study among a large cohort of HCWs in New York City (NYC) who were voluntarily tested reported a higher seroprevalence of antibodies (13.7%), which was correlated with the high COVID-19 burden [21].
- Similar to the present study, men were reported to be at a higher risk of SARS-CoV-2 infection in the studies conducted in Milan and Denmark [17,19]. Age was not found to be a significant predictor of seropositivity among HCWs in the NYC study, which corroborates the findings of this study [20]. Acquisition of SARS-CoV-2 infection locally from the community as the dominant mode of transmission among HCWs was also previously reported in a Dutch study [22].
- In the present study, the intake of an HCQ prophylaxis regimen did not have any protective effect on the risk of contracting the SARS-CoV-2 infection. Although some early observational studies indicated protective efficacy of HCQ against COVID-19, evidence generated from later clinical trials showed that daily HCQ does not prevent SARS-CoV-2 infection [23-25].
- In this study, the use of immunity boosters based on alternative systems of medicine, such as indigenous systems of Indian medicine and homeopathy, was associated with a significantly higher risk of seropositivity. The findings suggest that while immunity boosters probably offered no protection against the acquisition of SARS-CoV-2 infection, they might instill a false sense of security leading to reduced adherence to preventive measures, with increased risk of infection. Nonetheless, the potential impact of alternative systems of medicine in the management of COVID-19, including disease progression and severity, needs further evaluation.
- The strengths of the study include a large sample size and collection of data through face-to-face interviews with the employees of the largest state-run teaching hospital in Northern India catering only to COVID-19 patients. However, the study has several limitations. These include the use of a cross-sectional analysis, which precluded the assessment of any temporal or causal relationships, especially changes in seroprevalence with the progression of the pandemic, which would require a prospective study design. The study was conducted in a single institutional setting, limiting the generalizability of the findings. Furthermore, the employees who did not participate in the study may have differed in their sociodemographic and clinical characteristics, leading to potential selection bias. Finally, the data on adherence to preventive measures against COVID-19 were self-reported, which may have led to recall bias.
- In conclusion, the present study conducted at a teaching hospital in New Delhi observed that nearly 1 in 10 HCWs without any prior microbiological confirmation had detectable IgG antibodies to SARS-CoV-2. Considering that the doctors had the lowest seropositivity for SARS-CoV-2, whereas administrative staff had the highest seropositivity, the probability of HCW-to-HCW or patient-to-HCW transmission may be lower than the risk of transmission from other sources including the general population. However, non-pharmaceutical interventions were not associated with a reduction in the risk of acquisition of SARS-CoV-2, implying the necessity to improve training techniques among all hospital employees for maintaining the correct use of PPE on all occasions involving the risk of contracting the infection.
Discussion
Supplementary Material
-
Ethics Approval
The study protocol was approved by the Institutional Ethics Committee (F.1/IEC/MAMC/(77/05/2020/No 202). Written and informed consent was obtained from all the participants.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Availability of Data
The anonymized dataset that support the findings of this study will be made available by the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: PS, RC, RB, SS, PL, MD, SKB; Data curation: PS, RC, RB, PKB; Formal analysis: SB; Funding acquisition: Nil; Investigation: PS, RC, RB, PKB; Methodology PS, RC, RB, SB, MD; Project administration: PS, RC, PKB; Resources: PS, RC, SS, MD, SKS; Supervision: PS, SS, PL; Validation: PS, SS, MD; Visualization: SB; Writing–original draft: SB; Writing–review & editing: all authors.
-
Additional Contributions
Mr. Kumar Dushyant (research associate) contributed to creating the figure for the manuscript.
Article information
Data are presented as n (%) or OR (95% CI). Contact with body fluids (urine, blood, saliva) of a confirmed COVID-19 patient. Within a 1-m distance of a confirmed COVID-19 patient who was not wearing a mask/respirator.
IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; OR, odds ratio; CI, confidence interval; PPE, personal protective equipment; COVID-19, coronavirus disease 2019.
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727−33.ArticlePubMedPMC
- 2. Fauci AS, Lane HC, Redfield RR. Covid-19: navigating the uncharted. N Engl J Med 2020;382:1268−9.ArticlePubMedPMC
- 3. Ministry of Health and Family Welfare. Government of India. 2020 [Internet]. [cited 2020 Oct 8]. Available from: https://www.mohfw.gov.in/.
- 4. Suarez-Garcia I, Martinez de Aramayona Lopez MJ, Saez Vicente A, et al. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect 2020;106:357−63.ArticlePubMedPMC
- 5. Public Health England. COVID-19: guidance for maintaining services within health and care settings. Infection prevention and control recommendations [Internet]. Public Health England; 2020 [cited 2020 Oct 7]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881489/COVID-19_Infection_prevention_and_control_guidance_complete.pdf.
- 6. News: Coronavirus latest: WHO says health workers account for 10% of global infections. Deutsche Welle (DW) [Internet]. 2020 Aug 17 [cited 2020 Oct 7]. Available from: https://www.dw.com/en/coronavirus-latest-who-says-health-workers-account-for-10-of-global-infections/a-54208221.
- 7. Kapoor A, Kapoor KM. Covid-19 related deaths among doctors in India [Preprint]. Posted 2020;Sep 30 medRxiv 2020.09.28.20202796. https://doi.org/10.1101/2020.09.28.20202796.Article
- 8. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med 2020;173:362−7.PubMed
- 9. Dwivedi S. Delhi hospital treats 8,000 Covid patients: highest by state-run facility. The Times of India [Internet]. 2020 Oct 4 [cited 2020 Oct 7]. Available from: https://www.ndtv.com/delhi-news/delhi-lnjp-hospital-treats-8-000-covid-patients-highest-by-any-state-run-facility-in-india-2305037.
- 10. Indian Council of Medical Research. Revised advisory on the use of hydroxychloroquine (HCQ) as prophylaxis for SARS-CoV-2 infection (in supersession of previous advisory dated 23rd March, 2020) [Internet]. 2020 [cited 2020 Oct 7]. Available from: https://www.icmr.gov.in/pdf/covid/techdoc/V5_Revised_advisory_on_the_use_of_HCQ_SARS_CoV2_infection.pdf.
- 11. Sapkal G, Shete-Aich A, Jain R, et al. Development of indigenous IgG ELISA for the detection of anti-SARS-CoV-2 IgG. Indian J Med Res 2020;151:444−9.ArticlePubMedPMC
- 12. Aanensen DM, Huntley DM, Feil EJ, et al. EpiCollect: linking smartphones to web applications for epidemiology, ecology and community data collection. PLoS One 2009;4:e6968.ArticlePubMedPMC
- 13. Ministry of Health and Family Welfare, India. Containment plan: novel coronavirus disease 2019 (COVID 19) version 2 (updated 16.05.2020) [Internet]. New Delhi: Ministry of Health and Family Welfare, India; 2020 [cited 2020 Oct 7]. Available from: https://www.mohfw.gov.in/pdf/Containmentplan16052020.pdf.
- 14. Sharma N, Sharma P, Basu S, et al. The seroprevalence and trends of SARS-CoV-2 in Delhi, India: a repeated population-based seroepidemiological study [Priprint]. Posted 2020;Dec 14 medRxiv 2020.12.13.20248123. https://doi.org/10.1101/2020.12.13.20248123.Article
- 15. Khan SM, Qurieshi MA, Haq I, et al. Seroprevalence of SARS-CoV-2 specific IgG antibodies in District Srinagar, northern India: a cross-sectional study. PLoS One 2020;15:e0239303.ArticlePubMedPMC
- 16. Dave M, Vijayvargiya R, Poswal L, et al. Study of COVID-19 seroprevalence among healthcare workers at dedicated COVID hospital in southern Rajasthan. Indian J Clin Pract 2020;31:107−11.
- 17. Amendola A, Tanzi E, Folgori L, et al. Low seroprevalence of SARS-CoV-2 infection among healthcare workers of the largest children hospital in Milan during the pandemic wave. Infect Control Hosp Epidemiol 2020;41:1468−9.ArticlePubMed
- 18. Sotgiu G, Barassi A, Miozzo M, et al. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med 2020;20:203. ArticlePubMedPMC
- 19. Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis 2020;20:1401−8.ArticlePubMedPMC
- 20. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network: 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep 2020;69:1221−6.PubMedPMC
- 21. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA 2020;324:893−5.ArticlePubMedPMC
- 22. Kluytmans-van den Bergh MF, Buiting AG, Pas SD, et al. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open 2020;3:e209673.ArticlePubMedPMC
- 23. Chatterjee P, Anand T, Singh KJ, et al. Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19. Indian J Med Res 2020;151:459−67.ArticlePubMedPMC
- 24. Abella BS, Jolkovsky EL, Biney BT, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med 2021;181:195−202.ArticlePubMed
- 25. Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020;383:517−25.ArticlePubMed
References
Figure & Data
References
Citations

- Assessment of potential risk factors for COVID-19 among health care workers in a health care setting in Delhi, India -a cohort study
Mridu Dudeja, Aqsa Shaikh, Farzana Islam, Yasir Alvi, Mohammad Ahmad, Varun Kashyap, Vishal Singh, Anisur Rahman, Meely Panda, Neetu Shree, Shyamasree Nandy, Vineet Jain, Amitava Mukherjee
PLOS ONE.2023; 18(1): e0265290. CrossRef - Seroprevalence of IgG antibodies against SARS-CoV-2 in India, March 2020 to August 2021: a systematic review and meta-analysis
Nuzrath Jahan, Adarsha Brahma, Muthusamy Santhosh Kumar, Bhavani Shankara Bagepally, Manickam Ponnaiah, Tarun Bhatnagar, Manoj V Murhekar
International Journal of Infectious Diseases.2022; 116: 59. CrossRef - Risk Factors for COVID-19 Infection Among Healthcare Workers. A First Report From a Living Systematic Review and meta-Analysis
Tafadzwa Dzinamarira, Sphamandla Josias Nkambule, Mbuzeleni Hlongwa, Malizgani Mhango, Patrick Gad Iradukunda, Itai Chitungo, Mathias Dzobo, Munyaradzi Paul Mapingure, Innocent Chingombe, Moreblessing Mashora, Roda Madziva, Helena Herrera, Pelagia Makanda
Safety and Health at Work.2022; 13(3): 263. CrossRef - Seroprevalence of SARS-CoV-2 and Risk Assessment Among Healthcare Workers at a Dedicated Tertiary Care COVID-19 Hospital in Delhi, India: A Cohort Study
Pragya Sharma, Rohit Chawla, Saurav Basu, Sonal Saxena, Warisha Mariam, Pradeep Kumar Bharti, Shivani Rao, Neha Tanwar, Anisur Rahman, Mohammad Ahmad
Cureus.2021;[Epub] CrossRef


 PubReader
PubReader ePub Link
ePub Link Cite
Cite