Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 8(1); 2017 > Article
-
Meeting Report
The First Meeting of the National Control Laboratories for Vaccines and Biologicals in the Western Pacific in 2016 - Hokyung Oha, Jinho Shinb, Manabu Atoc, Xiao Mad, David Williamse, Kiwon Hana, Yang Jin Kimf, Hyunggoo Kangg, Kikyung Junga, Kentaro Hanadac, Masaki Ochiaic, Pham Van Hungh, Sangmi Parka,i, Chiyoung Ahna
-
Osong Public Health and Research Perspectives 2017;8(1):91-103.
DOI: https://doi.org/10.24171/j.phrp.2017.8.1.13
Published online: February 28, 2017
aBlood Products Division, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Cheongju, Korea
bWorld Health Organization, Regional Office for the Western Pacific, Manila, Philippines
cNational Institute of Infectious Diseases, Tokyo, Japan
dNational Institutes for Food and Drug Control, China Food and Drug Administration, Beijing, China
eDepartment of Pharmacology and Therapeutics, University of Melbourne, Parkville, VIC, Australia
fDepartment of Statistics, Sookmyung Women’s University College of Science, Seoul, Korea
gDepartment of Emergency Medicine, Hanyang University College of Medicine, Seoul, Korea
hNational Institute for Control of Vaccine and Biological, Ministry of Health Vietnam, Hanoi, Vietnam
iDepartment of Manufacturing Pharmacy, Chungbuk National University College of Pharmacy, Cheongju, Korea
- Corresponding author: Chiyoung Ahn, E-mail: cahn@korea.kr
Copyright © 2017 Korea Centers for Disease Control and Prevention
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
- The first meeting of the National Control Laboratories for Vaccines and Biologicals in the Western Pacific Region was held on September 1–2, 2016 in Seoul, the Republic of Korea. The meeting objectives were to share results of current research and to promote collaboration between the National Control Laboratories. To this end, we first discussed each country’s current status of research on quality control of biologicals. Next, we reviewed quality control of snake venom and antivenom production and the progress of a collaborative study on the Korean reference standard candidate for snake venom. We also discussed the establishment of the second regional reference standard antivenom and the characterization of the Vero cell genome landscape and its application to quality control. Moreover, we also reflected on the importance of collaboration among interested parties participating in this meeting. In conclusion, the meeting initiated networking between the national control laboratories in the Western Pacific region and paved the way to continue collaboration, which will eventually improve the region’s capacity for quality control of biologicals.
- Keywords: western pacific region; national control laboratory; meeting report; regional working reference standards; antivenom; collaborative study
- The International Standards (ISs) are a critical element of quality control (QC) testing of vaccines and biologicals. Their use allows monitoring of biologicals quality and international comparison of test results. A number of ISs have been established by several World Health Organization (WHO) collaborating centres (WHOCCs) since 1948, and serve as primary reference standards (PRS) for establishing secondary working reference standards (SWRS) at the national or regional level. Collaborative studies between laboratories are essential for calibrating these materials and determining their suitability for use. The WHO promotes and supports a regional collaborative approach to establishment of relevant regional working reference standards (RWRS) wherever possible.
- There are four active WHOCCs in the Western Pacific Region (WPR): the Therapeutic Goods Administration (TGA) in Australia, the National Institute of Infectious Diseases (NIID) in Japan, the National Institute of Food and Drug Safety Evaluation (NIFDS) in the Republic of Korea, and the National Institutes for Food and Drug Control (NIFDC) in China. These National Control Laboratories (NCLs) have been engaging in establishing SWRSs and participating in the development of ISs. This year, the member states are moving towards strengthening collaboration within the WPR NCLs. These WPR NCL network activities will support the continuity of efforts initiated by the above-mentioned meeting. Moreover, this meeting has the potential to contribute to strengthening the regional national regulatory authority (NRA) alliance to help achieve its primary goal of supporting strategies and programmes that develop and strengthen NRAs to ensure the quality of vaccines and other biologicals. The first meeting of the NCLs for vaccine and biologicals in the WPR was held on September 1 to 2, 2016 in Seoul, the Republic of Korea. The objectives of the meeting were to: 1) share the current status of research for QC of vaccine and blood products and explore topics for collaborative studies; 2) review the plan and progress of collaborative studies on snake venom and antivenom; 3) plan for collaborative studies on genomic sequencing to screen for adventitious agents; and 4) discuss strategies and agendas for NCL collaboration.
INTRODUCTION
- The two-day meeting was organized by the NIFDS, Ministry of Food and Drug Safety (MFDS) and supported by the WHO Regional Office for the Western Pacific (WPRO). The meeting was attended by 60 participants representing the NCLs of 4 countries (NIFDC, China; NIID, Japan; NIFDS, the Republic of Korea; and the National Institute for Control of Vaccines and Biologicals (NICVB), Vietnam), two manufacturers’ QC laboratories (Research Institute for Tropical Medicine [RITM], Philippines; Koreavaccine, Republic of Korea), one importer’s QC laboratory (Glovax, Republic of Korea), three academic institutions, (University of Melbourne, Australia; Hanyang University College of Medicine and Sookmyung Women’s University, the Republic of Korea), and WHO staff.
PARTICIPANTS
- 1. Session A: Sharing research findings among the NCLs
- To share research findings regarding QC of biologics and explore topics for collaborative studies, NCL representatives from NIFDS, NIFDC, NIID, and NICVB presented the current status of research for QC of vaccine and blood products.
- Dr. Chulhyun Lee (NIFDS, the Republic of Korea) provided the status of establishing national reference standards (NRSs), including pertussis antigens for enzyme-linked immunosorbent assay (ELISA) (pertussis toxin [PT], filamentous heamagglutinin [FHA], pertactin), pneumococcal conjugate mixtures, an inactivated Vero-Beijing-1 Japanese encephalitis vaccine, and a varicella vaccine.
- Dr. Lee said that coating antigen candidates for ELISA will be used in a mouse immunogenicity test, which is an alternative to the modified mouse intracerebral challenge assay (MICA) for determining the potency of acellular pertussis (aP) vaccines. These candidate materials were prepared in liquid form and produced by the manufacturer Green Cross, from the Republic of Korea. Each vial of coating antigen contains 0.1702 mg/mL of PT, 0.1585 mg/mL of FHA, and 0.1844 mg/mL of pertactin.
- Moreover, the NRS candidate for the pneumococcal conjugate vaccine composed of thirteen pneumococcal polysaccharide conjugate mixtures is being manufactured by SK Chemical and will be used in a polysaccharide content test.
- There is insufficient stock of the 2nd NRS (code No. 08/027, MAV/06 strain) of the varicella vaccine and this requires replacement with a 3rd NRS. The candidate materials of the 3rd NRS prepared from the Oka strain were manufactured by SK Chemical. The virus content of this candidate material was verified by comparison with the 2nd NRS, and results showed an acceptable titre of more than 4.50 Log plaque forming unit (PFU) per 0.5 mL. Other quality test results met the acceptance criteria. A collaborative study to assign the virus content will be underway by the end of this year.
- Dr. Kikyung Jung (NIFDS, the Republic of Korea) outlined current studies on the development of a QC test method for blood products. The current projects are:
Establishing a snake venom NRS for use in an antivenom potency test;
Studying international harmonization and establishing a test method for thrombin generation; and,
Establishing an in vitro potency test for anti-tetanus immunoglobulin as an alternative to animal tests.
- Dr. Jung also spoke about the monocyte activation test (MAT), which uses rabbit peripheral blood mononuclear cells (PBMCs) to replace the rabbit pyrogen test. The MAT using rabbit PBMCs was characterized with several exogenous pyrogens, including lipopolysaccharide, zymosan, and the Saccharomyces cerevisiae cell wall, to show a dose-dependent increment of endogenous pyrogens, such as interleukin (IL)-6, IL-1β, and tumor necrosis factor-α in rabbit PBMCs. Although the current European Pharmacopoeia recommends that the MAT use human PBMCs, procuring uniform human PBMCs is not feasible in the Republic of Korea. Therefore, these results support the plausibility of alternative approaches to the rabbit pyrogen test.
- Dr. Xiao Ma (NIFDC, China) provided an overview of his organization and its functions, the staff-in-charge, and the lot release testing of biologics. Annually, almost 5,000 lots of fifty-one categories of vaccines from forty manufacturers, equivalent to approximately one billion doses, are examined for their quality by the NIFDC.
- Dr. Ma said the NIFDC has conducted various studies to develop an academic programme of biological product testing and ensure the specifications of biological products. Among them, current studies by the Division of Diptheria-Tetanus-Pertussis Vaccine and Antitoxin include:
Evaluating the efficacy of the pertussis vaccine in different mouse strains;
Evaluating the use of an antibody titre test as an alternative to the MICA for pertussis vaccines;
Investigating adenylate cyclase toxin in the aP vaccine by ELISA;
Evaluating novel methods for testing pertussis toxicity (enzymatic high performance liquid chromatography [HPLC], Chinese hamster ovary [CHO] clustering, fetuin-binding ELISA); and,
Researching the polymer content of toxoid by HPLC.
- Dr. Ma also presented the complete genome sequence of Bordetella pertussis strain CS, which was isolated from an infant in 1951 in Beijing and is widely used as a vaccine strain for producing an aP vaccine in China. The genome sequence was compared with that of the Tohama I strain [1]. The complete genome of the CS strain is encoded in a circular 4,124,236 bp chromosome, with an average GC content of 67.3%, and it has 3,456 protein-coding sequences, with an average size of 327 amino acids, 51 tRNA genes, and three rRNA operons [2]. Compared with the Tohama I strain, two large fragments (> 10 kb) were exclusively present in the CS strain. These fragments are associated with the transcriptional regulator system, metabolism, mobile elements, and the restriction modification system [2]. The complete genome sequence of B. pertussis CS will inform future bioinformatic and phylogenetic studies.
- Dr. Masaki Ochiai (NIID, Japan) presented the current status of research for QC of vaccines. The current research studies at the NIID are:
Evaluating a single radial immune-diffusion (SRID) assay to accurately measure the hemagglutinin (HA) content of two influenza B virus components of the quadrivalent influenza vaccine (QIV);
Developing an antigen ELISA as an alternative to the in vivo potency test for the inactivated Japanese encephalitis vaccine;
Evaluating a D-antigen ELISA as an alternative to the in vivo potency test in rats for the Sabin-based inactivated poliomyelitis vaccine;
Developing an antigen ELISA as an alternative to the in vivo potency test in mice for hepatitis A & B vaccines;
Developing a sensitive in vitro assay to detect residual viable rabies virus in the inactivated rabies vaccine; and,
Refining the histamine sensitization test (HIST) and developing alternatives to the HIST for aP vaccines.
- Dr. Ochiai stated that the NIID evaluated a suitable SRID assay to precisely measure the HA content in two influenza B virus components in the QIV because antigens from these two viral lineages cross-react with antiserum raised against a different lineage of HA in some cases. Cross-reactivity of the two influenza B virus components in the SRID assay varied depending on the virus strain and/or manufacturer. To overcome this obstacle, an alternative SRID assay with mixed-standard antigens (stdAgs) from both lineages of influenza B virus was proposed; however, this method is still not fully characterized. Therefore, SRID assays using either the single-stdAg or mixed-stdAgs were examined to establish a suitable procedure for accurately determining the HA content in prototype QIVs (2015/16 season) produced by four Japanese manufacturers. As a result, regardless of the manufacturer, the precise HA content of the Phuket strain (B/Yamagata lineage) could be measured by the SRID assay using either the single-stdAg or mixed-stdAgs, but the HA content of the Texas strain (B/Victoria lineage) was accurately measured only using the mixed stdAgs. It is necessary to establish a suitable procedure for each combination of stdAg, antiserum, and manufacturer for accurate measurement of the HA content in QIVs.
- Dr. Ochiai also discussed the development of alternative methods, including an enzymatic HPLC assay, a carbohydrate binding assay, and a CHO cell clustering assay, to the murine HIST, which is used to measure residual bioactive pertussis toxin in aP vaccines. Alternative in vitro assays should ideally allow direct comparison with the existing in vivo assay, but such a comparison is generally challenging. However, alternative in vitro assay methods should be required to be relevant to the existing in vivo assay method. An alternative in vitro assay method must at least detect the difference between failed lots and passed lots to a level comparable to that which is detectable by the existing in vivo assay to maintain the quality assured by the existing test.
- Dr. Pham Van Hung (NICVB, Vietnam) outlined the regulatory system, lot release, and laboratory access to vaccine and biological products at the NICBV/NCL, Vietnam. As explained by Dr. Hung, plans for future work at the NICBV/NCL are:
Strengthening the ability of the NICVB/NCL to participate in the NCL network;
Implementing an NICVB-Lab system reaching the WHO-GLP, ISO 17025, 15189 supported by the Ministry of Health;
Improving the ability to research, establish, and manage NRSs; implementing NRS projects; and collaborating with the regional NCLs to develop RWRS for vaccines and biologics;
Establishing a training plan for new staff about proper research techniques, the quality management system, and QC techniques in new vaccines;
Organizing an annual harmonization workshop on lot release and QC testing methods between the NICVB and the manufacturers; and,
Ensuring that all equipment will be calibrated and maintained by NICBV staff.
- 2. Session B: Snake venom and antivenom
- Dr. David Williams (University of Melbourne, Australia) gave a presentation on the introduction of WHO’s Guidelines for the Production, Control, and Regulation of Snake Antivenom Immunoglobulins and an online database identifying the worldwide distribution of medically important venomous snakes and their existing antivenoms. Snake antivenom is the only specific treatment for envenoming by snakebite [3]. Antivenom can prevent or reverse many of the effects of snakebite envenoming, and plays a crucial role in minimizing mortality and morbidity (http://www.who.int/bloodproducts/snake_antivenoms/en/). Dr. Williams said that very few countries currently have access to snake venoms of adequate quality for antivenom manufacturing. In addition, poor data have led to the underestimation of antivenom needs by national health authorities, leading to low demand for manufacturers to produce antivenom and implement appropriate procurement and antivenom distribution strategies. For these reasons, he explained, the WHO Guidelines are intended to inform NRAs and manufacturers in their efforts to improve the worldwide production of safe and effective antivenoms. Therefore, this document describes the fundamental requirements for the Good Manufacturing Practice-like production of snake antivenom immunoglobulin preparations for therapeutic use. Moreover, the WHO’s global database of venomous snakes, including maps and an image library, was created to raise awareness of the geographical distribution of the most medically important venomous snakes. Information about the species that are priorities for antivenom production is available in the Annex I of the Guidelines. The WHO Guidelines may support public health officers, procurement agencies, regulators, and manufacturers involved in decision-making related to the preparation and use of appropriate antivenoms and assist health workers during clinical management of snakebite envenoming.
- Dr. Williams also reported that a revision of the WHO antive-nom Guidelines was underway currently and that a final revised version was being presented to the ECBS in October 2016 for establishment. The key changes made in the revision are:
Updates to lists of medically important snakes to reflect the discovery of new species and changes to nomenclature;
Revision of methodologies for serpentariums producing venoms to emphasize traceability and QC, including the recommendation to discontinue the use of wild-caught snakes for ethical and QC reasons;
Need for national reference venom collections that are independent of manufacturers;
A recommendation for research into new adjuvants;
Updates to tables of known equine viruses;
Greater emphasis on the specific health controls of donor animals prior to and during bleeding sessions;
Redrafting QC and preclinical testing chapters to eliminate redundancy in describing lethality testing in animal models, strengthen messages regarding the ethical use of animals in experiments, and update stability study recommendations;
Inclusion of antivenomics as an additional preclinical testing methodology that can supplement conventional approaches; and,
Updates to the clinical assessment chapter and expanded information on the role of regulatory authorities in antive-nom production.
- Dr. Hyunggoo Kang (Hanyang University, the Republic of Korea) discussed the current trends of snakebite management in the Republic of Korea. According to a report by the WHO, approximately 5 million snakebites occur each year, resulting in up to 2.5 million envenomings [4], at least 100,000 deaths, and three times as many amputations and other permanent disabilities [4–6]. According to the Korean statistical information service (http://kosis.kr), more than 600 cases of snake envenoming occur annually, but no deaths have occurred in the last 10 years in the Republic of Korea. Four species of venomous snakes reside in the Republic of Korea: Gloydius brevicaudus, Gloydius intermedius, Gloydius ussuriensis, and Rhabdophis tigrinus. Dr. Kang summarized the characteristics of venomous snakes found in the Republic of Korea [7] (Table 1). Dr. Kang stated that the LD50 value for the venom of G. ussuriensis was 8.68 μg per mouse (ICR; body weight, 17 ± 1 g), which is the most toxic among the three Gloydius spp. The LD50 values of G. intermedius and G. brevicaudus venoms were 10.28 μg and 45.8 μg per mouse, respectively. The absolute lethal dose (LD100) of G. intermedius and G. brevicaudus was 300 μg per mouse, and that of G. ussuriensis was 200 μg per mouse. The venom of G. ussuriensis also had the strongest haemorrhagic activity, a minimum haemorrhagic dose (MHD) of 1.36 μg per rabbit (New Zealand White; body weight, 2.5 ± 0.3 kg), and the MHD values of G. brevicaudus and G. intermedius were 2.39 μg and 2.39 μg per rabbit, respectively [8]. However, their venoms are not very toxic, and systemic bleeding resulting from these snakebites is rare. He also summarized the frequency of different grading’s of envenoming among patients with snake-bite in the Republic of Korea (Table 2 [9–17]). Korean snake venoms (Gloydius and Rhabdophis spp.) mainly cause haematological abnormalities of thrombocytopoenia and venom-induced consumption coagulopathy. However, he noticed that there is no central policy regarding the standard treatment of venomous snakebites, and no guidelines for antivenom injection and dosage vary between hospitals in the Republic of Korea.
- Ms. Chari Jane G. Rosales (RITM, the Philippines) described all the QC tests performed on purified and concentrated cobra antivenom produced by the Biologicals Manufacturing Division of RITM in the Philippines. The Philippine cobra (Naja philippenensis) is a stocky, highly venomous species of spitting cobra native to the Northern region of the Philippines. They can be found on the islands of Luzon, Mindanao, Catanduanes, and Masbate. The average length of this species is 1.0 m. The venom of the Philippine cobra is a potent postsynaptic neurotoxin that causes respiratory paralysis. Ms. Rosales said that the Biologicals Manufacturing Division of RITM is responsible for producing purified liquid cobra antivenom in accordance with the requirements of the local regulatory authority. The QC tests performed by RITM as part of the batch release include the rabbit pyrogen test, LAL test, abnormal toxicity test, and potency test. She also said that a stability test should be performed to determine the stability and shelf life of the antivenom, proving that the antivenom remains stable and efficacious until the expiry date.
- Dr. Manabu Ato (NIID, Japan) provided a brief overview of the lot release testing on antivenoms at NIID. He said that three types of antivenoms exist in Japan: Habu antivenom from Protobothrops flavoviridis, Mamushi antivenom from Gloydius blomhoffii, and Yakakagashi antivenom from R. tigrinus. All antivenoms are manufactured by Kaketsuken, which has its own equine farm to ensure full traceability and health surveillance of animals used in antivenom production programmes. Dr. Ato said that NIID performs the lot release testing for the lyophilized Habu and Mamushi antivenoms but not for Yamakagashi antivenom because it is an unapproved drug used only for clinical studies.
- Dr. Ato also discussed a previous collaboration to produce a RWRS of antivenom (code no. 011201) conducted by Japan, China, and the Republic of Korea in 2004 [18]. No ISs have been appropriate in the antivenom field due to the existence of considerable variation among different snake species. However, Gloydius spp., that produce similar venoms, inhabit East and Central Asia, including Japan, China, and the Republic of Korea. At a working group meeting involving three NCLs held in China in 2002, the collaborative preparation of a RWRS of Gloydius spp. antivenom was discussed. The potency of the RWRS candidate was examined against the Japanese Mamushi antivenom (G. blomhoffii) NRS (Lot C-48, anti-lethal titre: 2.100 U/vial; anti-haemorrhagic titre: 3,300 U/vial, NIID). In 2006, the RWRS of antivenom was established and used in QC of antivenom products at NIID, NIFDC, and NIFDS. This study was the first to establish a RWRS as recommended by the WHO [19]. Dr. Ato said that further studies will be needed to validate the toxicity and quality of local venoms, to evaluate the neutralizing activity of the antivenom against locally obtained snake venoms, to develop in vitro alternative potency tests, and to comply with animal health monitoring and viral validation studies according to the WHO antivenom guidelines.
- Dr. Byung-Hwa Lee (Koreavaccine, the Republic of Korea) presented an overview of the manufacturing process of the Korean snake venom NRS candidate, originated from G. brevicaudus siniticus from China. Currently, Koreavaccine produces Kovax Freeze-dried G. brevicaudus siniticus antivenom (previously labelled as Agkistrodon halys antivenom), which is manufactured from final bulk supplied from Shanghai Serum Biotech.
- Dr. Lee said that Koreavaccine also manufactured 1,563 vials of a lyophilized venom candidate using venom from Chinese G. brevicaudus siniticus. The formulation (1 mL per vial) contains 20 mg G. brevicaudus siniticus venom, 8.5 mg NaCl, 10 mg sucrose, 20 mg human serum albumin, and Tris-HCl buffer (0.01 M, pH 8.0) (Table 3). To evaluate the immunological characteristics associated with the venom candidate and the antivenom RWRS, Koreavaccine performed several preliminary tests, including an immunodiffusion assay, immuno-electrophoresis, and animal testing. Pilot studies were also conducted to find the optimal conditions for lyophilizing the candidate material (Table 4). To ensure the quality of lyophilized venom candidate, Koreavaccine performed QC tests and the quality of the venom candidate was verified to meet QC standards.
- Dr. Kiwon Han (MFDS, the Republic of Korea) provided an overview of an international collaborative study for establishing the second Korean G. brevicaudus siniticus snake venom NRS. The aim of this study was to assign the potency value of venom NRS candidate against the antivenom RWRS by measuring the murine anti-lethal titres and the lapine anti-haemorrhagic titres of the antivenom against this venom. This study began in May 2016, and the data will be analysed for inclusion in the study report, which should be completed by March 2017. The study participants were initially scheduled to submit their data by the end of August 2016 for analysis, but submission has been postponed to the end of December 2016 to accommodate additional participants. Six laboratories took part in determining anti-lethal titres and/or anti-haemorrhagic titres, including laboratories from Japan (1), China (1), Indonesia (1), and the Republic of Korea (3), comprising 1 manufacturer, 1 medical research laboratory, and 4 regulatory laboratories. For this collaborative study, samples of the antivenom RWRS (code no. 011201), the venom candidate, and the test protocols were supplied to the six laboratories. The participants had the option of choosing to conduct one or both of the testing methods offered. Details of the methods used in the study were previously described [18].
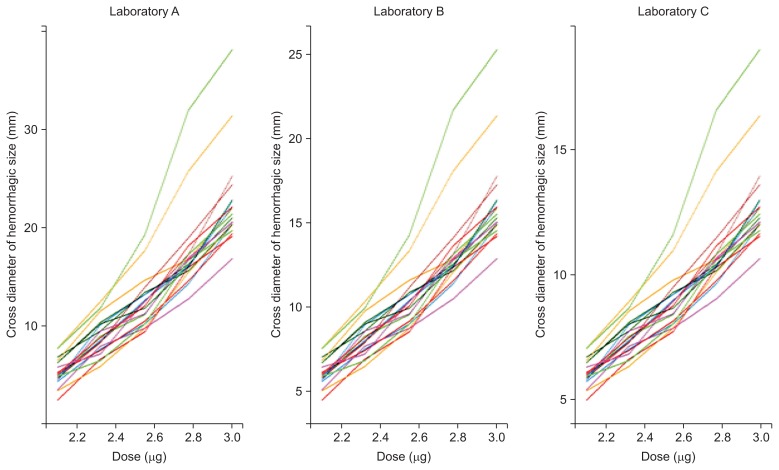
- Prof. Yang Jin Kim (Sookmyung Women’s University, the Republic of Korea) presented a statistical analysis of G. brevicaudus siniticus venom MHD test results obtained from three domestic laboratories. After 24 hours of five dilution injections into the shaved back of a rabbit, the cross diameter of haemorrhagic spots on excised skin was measured. For estimating the haemorrhagic dose in this test, she proposed two basic statistical methods depending on quantitative responses: the parallel line method and slope ratio method. The results of the parallel line method are linearly related to the log dose, whereas the results of the slope ratio method are linearly related to the dose itself. No significant difference was found between the three laboratories, and the mean haemorrhagic dose was 1.099 μg by the parallel line method (p = 0.411) and 1.142 μg by the slope ratio method (p = 0.235). Dr. Kim also proposed a mixed effect model as an alternative when several variations occur between subjects [20]. When comparing drug responses in a biological system, there are several factors that affect the response, including biological unit, batch, operator, and sample preparation process. A mixed effect model was applied to estimate the different variation components affecting haemorrhagic dose data (for a lesion of 10 mm cross diameter) from 3 domestic laboratories. Figure 1 shows each spaghetti plot, which tracks responses over the effect of the dose [21]. Every rabbit had a different starting point and a different slope over dose. This diversity results in variation among subjects. To model such possible variation, a mixed effect model was applied. In more detail, the fixed effects of the model are log (dose), and the random effect is the rabbit itself. The results of the three models are shown in Table 5. According to a goodness-of-fit test, the third model, a mixed effect model with random intercept and random slope, was the best fit model among three laboratories. Based on the third model, the MHD values determined by each laboratory were 1.077 μg (95% CI, 0.786–1.476 μg; Laboratory A), 1.001 μg (95% CI, 0.700–1.431 μg; Laboratory B), and 1.124 μg (95% CI, 0.955–1.323 μg; Laboratory C).
- 3. Session C: Proposal for a collaborative study on antivenom
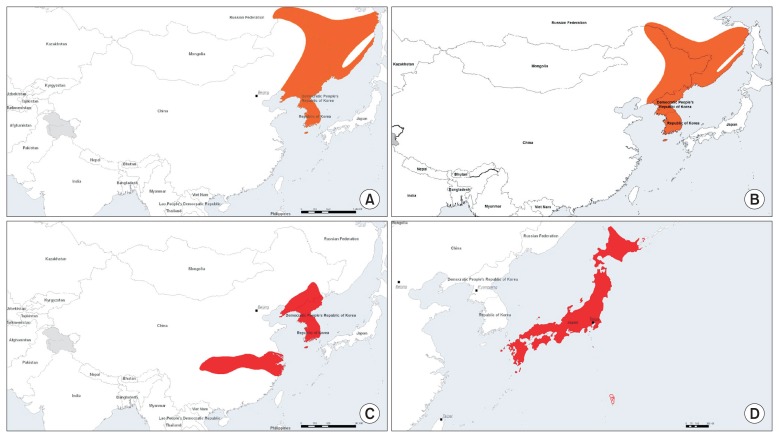
- NIFDS proposed a project to replace the antivenom RWRS, which was the first antivenom RWRS in three countries (China, Japan, and the Republic of Korea). This RWRS was established in 2006. The three countries’ NCLs equally split the quantity of antivenom RWRS. NIFDS expects to exhaust their stock within 4 years, and replacing the antivenom RWRS is a high priority. However, there are several key considerations to discuss prior to beginning the project. First, the total quantity of demand (vials needed per NCL per annum) has to be clarified. Currently, NIFDS has less than 100 vials of the original RWRS, and uses approximately 30 vials per year, emphasizing the need to have a replacement available within 2 to 3 years. On the other hand, NIID and NIFDC have ~170 vials and 100 vials and use 10 vials and 3 to 4 vials each year, respectively, sufficient to last at least another decade. Therefore, replacing the antivenom RWRS was agreed to for the future, but without urgency. Moreover, the reasons for using the RWRS in each country have to be clarified. The first antivenom RWRS had originally been used in routine QC tests as a SWRS at NIFDC, NIID, and NIFDS [18]. However, NIFDC had also established an antivenom NRS based on the first antive-nom RWRS; thereafter, the antivenom RWRS was used as a PRS, not a SWR. Therefore, when replacing the antivenom RWRS, the intended usage should be considered with priority to sustain the stock of antivenom RWRS. As a proposal, the second antivenom RWRS could be designated as a PRS, and not for use in routine assays. Instead, the RWRS would be supplied as a PRS for use as a basis in preparing SWRS. Another consideration is about the selection of snake venom for antivenom RWRS manufacturing. The first antivenom RWRS originated from G. brevicaudus siniticus was manufactured by Shanghai Serum Biotech. For the purposes of antivenom production prioritization, the WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins [3], classified medically important snake species into one of two categories (refer Annex 1 of the Guidelines). Species listed in category 1 are considered as being of highest priority for antivenom production, whereas those in category 2 are considered of secondary priority. There are 4 species of Gloydius spp. distributed in China, Japan, and the Republic of Korea: G. ussuriensis (Figure 2A), G. intermedius (Figure 2B), G. brevicaudus (Figure 2C), and G. blomhoffii (Figure 2D). Where a species is considered to be of category 1 importance it is shown is red, while category 2 priority is shown in orange (Figure 2). Gloydius spp. venoms have previously been shown to have very similar immunological characteristics [18]. Koreavaccine who manufacture antivenom for treating Gloydius spp. envenoming in the Republic of Korea import final bulk antivenom produced in China (by Shanghai Serum Biotech), and have conformed the potency of this antivenom which is prepared against venom obtained from captured G. brevicaudus siniticus in Zhejiang province China. Furthermore, G. brevicaudus siniticus falls into category 1 in China and the Republic of Korea, while G. blomhoffii falls into category 1 in Japan. The Japanese manufacturer produces antivenom against venom obtained from G. blomhoffii, whereas manufacturers in China and the Republic of Korea produce antivenom using venom from G. brevicaudus siniticus. Therefore, further discussion regarding the venoms to be used to establish the next antivenom RWRS is necessary. In developing a new RWRS it is essential to establish a new antivenom design that takes into account the selection of appropriate snake venoms, incorporates evaluation of their composition, and clinical relevance as causes of snakebite envenoming regionally. At any rate, the stock at NIFDS will likely be exhausted within 3 to 4 years, unless a strategy for redistribution of stock from the first antivenom RWRS is devised cooperatively to ensure that each country has adequate supplies for several more years. Alternately the establishment of a Korean antivenom NRS should be considered. NIFDS would need to secure a NRS candidate from a manufacturer of a licenced product. It should assess trial formulations for bioactivity using the ED50 venom lethality neutralization test and the MHD50 test for median effective dose against venom MHD for antivenom NRS candidate before proceeding to a definite fill. A Korean antivenom NRS should ideally be a product that is manufactured using snake venom from G. brevicaudus collected in the Republic of Korea, however the current product is manufactured from bulk made in China from the venom of G. brevicaudus siniticus collected in that country. After manufacturing the NRS candidate, and in light of scarcity of competent domestic testing laboratories, NIFDS will propose organizing a collaborative study to characterize and establish the neutralizing potency of the new antivenom NRS candidate in the next WPR NCL meeting to be held in 2017. A collaborative study will be organized involving manufacturers and expert laboratories in the WPR, and the establishment of Korean antivenom NRS is expected in 2019.
- At present NIFDS undertakes two assays of activity in assessing each batch of antivenom prior to batch release. The first is the ‘potency test’ which determines the ED50 of the antivenom, that is the dose required to protect 50% of mice tested from the lethal activity of the venom NRS. Next, the MHD50 of the antivenom, being the dose that reduces the diameter of haemorrhagic lesions by 50% compared to those induced in rabbits who receive a control solution is determined [22]. NIFDS have proposed the streamlining of the antivenom testing methods and statistical analysis. Most regulatory authorities, such as the WHO [3] and those in Europe [23], the United States [24], and China [25], require only the antivenom median neutralizing potency test, whereas authorities in Japan [26] and Korea [22] perform both tests. It should however be noted that, in vivo animal testing causes considerable suffering to test subjects however there are no in vitro replacement tests available, and laboratories should address welfare issues by working to validate protocols that incorporate appropriate analgesic strategies. The alternative at present is to develop strategies that reduce the number of animals used in such experiments as part of a commitment to the 3R Principles (Replacement, Reduction and Refinement). Therefore, in the Republic of Korea and in Japan, streamlining and rationalization of the antivenom median neutralizing potency test method should be considered.
- Furthermore, to measure the median neutralizing potency of antivenom, alternative statistical models to fit the experimental results should be considered and validated. NIFDS and the Korean antivenom manufacturers have used a Reed-Muench model as a specific analysis for the median neutralizing potency tests of antivenom. The Reed-Muench model is a simple method for determining the LD50 or ED50 in testing, that is, the concentration of a test substance that produces an effect of interest in half of the test subjects. However, this model is problematic because of difficulties in obtaining confidence intervals and requires a probability distribution. Meanwhile, a probit model and a logit model are types of regression, where the dependent variable can take only two values. These models could yield a more reliable analysis in binomial response variables than a Muench model. NIID has also used a probit model in a median neutralizing potency test for antivenom, and improving statistical modelling was agreed as being very important to QC testing in biologicals. A further important consideration is the need to take into account the specific approach of the manufacturer or NCL in establishing appropriate statistical modelling methodologies to use with the experimental results.
- 4. Session D: Proposals for future collaborative studies
- Dr. Kentaro Hanada (NIID, Japan) presented an overview of approaches to determine the genome landscape of the African green monkey kidney-derived Vero cell line, which is used to produce various types of vaccines, including those for poliovirus, Japanese encephalitis virus, rotavirus, and influenza virus. Dr. Hanada provided a draft sequence of the whole genome of the Vero cell line after massively parallel sequencing of genomic DNA and karyological and RNA-seq analyses [27]. Primary culture of kidney tissue from an African green monkey began on 27 March 1962 at Chiba University in Japan, after which several continuous cell sub-lines were obtained following passaging for several months, and a sub-line was then chosen as the standard Vero cell line [28]. The name, Vero, comes from “Verda Reno”, which means “green kidney” in Esperanto (https://en.wikipedia.org/wiki/Vero_cell). Vero cells are highly susceptible to various types of viruses and toxins, including simian polyoma virus SV-40, measles virus, rubella virus, arboviruses, adenoviruses, diphtheria toxin, heat-labile enterotoxins, and Shiga-like toxins. Moreover, Vero cells have pseudo diploid karyotypes and are non-tumourigenic when a cell passage is not prolonged. Therefore, the Vero cell lineage has been successfully utilized as a cell substrate for human vaccines. He said that the whole-genome sequences of the Vero cell line provide invaluable basic information for various purposes, including the development of new QC tests for the Vero cell lineage (Table 6).
- Dr. Hanada proposed a collaborative study on the validation of a novel genomics-based test methods for identifying adventitious agents by deep-sequencing or metagenome analysis of the master cell bank and working cell bank of vaccines. To assess whether such a new test could be a WHO-approved alternative to laborious conventional tests, a comparative analysis between the current and new test method must be performed using a known quantity of adventitious agents in intentional spiking studies for validation. He also proposed a collaborative study to determine genome landscapes of cell substrates for human vaccines and biologicals. Many animal cell lines have been and will be approved as the cell substrates for the production of biologicals. Their genome landscapes will be crucial basic information of use in QC.
- Dr. Naery Lee (NIFDS, the Republic of Korea) proposed a collaborative study on the suitability of egg-based influenza SRID reference reagents for use in cell culture-based vaccine for SRID HA content testing. Currently, there are two cell culture-based vaccines available globally: SKY cell flu (SK chemical) and Flucelvax (Novartis). SRID serum references against egg-based influenza vaccine viruses are available from Influenza Essential Regulatory Laboratories; however, international SRID serum references against cell culture-grown influenza vaccine viruses are not available. The egg-based SRID reference underestimates the HA content of cell-based vaccines, and the antiserum that is used to test cell-based vaccines should therefore be raised from Madin-Darby canine kidney epithelial cell grown flu antigen.
- As this problem is relevant only to the countries where cell-based seasonal flu vaccines are licenced, there was little interest in pursuing collaborative research studies by participants from countries where the products are not used. However, one of the Japanese participants stated that it is scientifically rational to use a flu HA antigen purified from virus particles produced in cell culture and not from egg-based flu virus as the HA standard for cell-based flu vaccines.
- 5. Session E: The ath forward: Western Pacific Laboratory Network
- In light of the long history of global and regional collaborations among Member States, the NCLs for vaccines and biologicals in the WPR have been active in supporting: a) establishment of RWRSs for official QC testing of vaccines and biologicals; b) facilitation of inter-laboratory collaborations on new/improved QC test methods; c) sharing of the regulatory research agenda; d) sharing the best practices on NRA’s lot release; e) facilitation of proficiency test studies to improve competency for QC of vaccines and biologicals; and f) identification of the need to expand testing capacity in countries with limited quality surveillance of vaccines and biologicals. In a rapidly changing world, the nature of collaboration between NCLs requires revisiting, in particular, the scope and collaboration agenda. Participation in the network should be open and flexible. Academic institutions and manufacturers’ QC laboratories should be part of the network. The efforts of the WPR NCL collaboration must avoid duplication of work, except where otherwise justified, and focus on establishing solid ground towards synergy, driven by the desire to add value.
- The NCLs who participated in this meeting are well positioned to continue research focused on snake antivenom standardization. An inactivated Japanese encephalitis vaccine containing the Beijing-1 strain propagated in Vero cells has been licenced in the Republic of Korea. The Beijing-1 strain was originated from the Beijing-Handai strain. A proposal for the establishment of the WHO IS Vero Beijing-1 JE vaccine has already been endorsed by WHO’s ECBS, and a candidate material is ready to be studied by NCLs and manufacturers’ QC laboratories in SEAR and WPR. The upcoming SEAR NCL network meeting will take place in December 2016, and all concerned NCLs are encouraged to attend or be informed of future collaborative studies arising form that meeting.
MEETING PRESENTATIONS AND DISCUSSION
- The September 2016 Seoul NCL meeting shared information on the current topics related to QC research in biologics and discussed the latest research studies and developments on standardizing a snake antivenom raised from the venom of G. brevicaudus siniticus which is of common interest to China, Japan, and the Republic of Korea. Participation by academic, industry, and NCL representatives provided the opportunity to share important insights into the current similarities and differences surrounding antivenom potency testing and the establishment of an antive-nom RWRS. Continuing collaboration among interested parties who participated at this meeting will contribute to improving the availability of safe, effective biologics of assured quality. The participants agreed on a follow-up meeting next year to review the progress on research subjects relating to snake antivenom and blood products.
CONCLUSION
-
Acknowledgements
- The authors extend their sincere appreciation to all participants in the meeting of the Western Pacific NCLs for their contributions to the meeting conclusions and recommendations. The members of the NIFDS are acknowledged for their contributions in planning the meeting, and all the invited experts are acknowledged for their contributions in breakout discussions and workshop proceedings. This meeting was financially supported by the MFDS (MFDS 093-2100-2131-300). A collaborative study on value assignment of snake venom reference candidate was supported by a grant from the scientific research programme (16201MFDS201) at NIFDS, MFDS. Travel expenses of two participants from the NIID were mainly supported by grant-in-aid from the Ministry of Health, Labour and Welfare, Japan (H27-IyakuA-Ippan-004).
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported. The authors alone are responsible for the views expressed in this report and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. The mention of specific companies or of certain manufacturers’ products dose not imply that they are endorsed or recommended by the participating national control laboratories in preference to others of a similar nature that are not mentioned.
Article information
- 1. Parkhill J, Sebaihia M, Preston A, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet 2003;35:32−40. https://doi.org/10.1038/ng1227. PMID: 10.1038/ng1227. PMID: 12910271.ArticlePubMed
- 2. Zhang S, Xu Y, Zhou Z, et al. Complete genome sequence of Bordetella pertussis CS, a Chinese pertussis vaccine strain. J Bacteriol 2011;193:4017−8. https://doi.org/10.1128/JB.05184-11. PMID: 10.1128/JB.05184-11. PMID: 21622744.ArticlePubMedPMC
- 3. World Health Organization. WHO guidelines for the production control and regulation of snake antivenom immunoglobulins [Internet]. Geneva: World Health Organization; 2010. [cited 2017 Jan 1]. Available from: http://www.who.int/bloodproducts/snake_antiven-oms/snakeantivenomguide/en/.
- 4. World Health Organization. Rabies and envenomings: a neglected public health issue. Report of a consultative meeting. Geneva: World Health Organization; 2007.
- 5. Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ 1998;76:515−24. PMID: 9868843.PubMedPMC
- 6. Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 2008;5:e218https://doi.org/10.1371/journal.pmed.0050218. PMID: 10.1371/journal.pmed.0050218. PMID: 18986210.ArticlePubMedPMC
- 7. Lim H, Kang HG, Kim KH. Antivenom for snake bite in Korea. J Korean Med Assoc 2013;56:1091−103. https://doi.org/10.5124/jkma.2013.56.12.1091. PMID: 10.5124/jkma.2013.56.12.1091.Article
- 8. Yoo CK, Kim YM, Park MY, et al. Determination of venom toxicity and standardization of venom and antivenin of Korean Agkistrodon spp. Korean J Vet Public Health 1999;23:135−42.
- 9. You KM, Kwon WY, Kwon TH, et al. Optimal dose of antivenin for asymptomatic or minor envenomation patient with Korean viperidae injuries. J Korean Soc Emerg Med 2013;24:420−7.
- 10. Kim DH, Choe SM, Oh YM, et al. Clinical significance of delayed reevaluation in initial symptoms following snakebite injury. J Korean Soc Clin Toxicol 2009;7:97−104.
- 11. Park EJ, Yoon SK, Ahn JH, et al. Systemic complications occurring after Korean venomous snake bite, with focus on hematologic and neurologic complications. J Korean Soc Clin Toxicol 2009;7:90−6.
- 12. Jin SC, Lee JW, Yang SJ, et al. Consideration of factors associated with complications and systemic symptoms of snake bites. J Korean Soc Emerg Med 2008;19:686−96.
- 13. Jun DH, Lee DP, Choi WI. Initial assessment of the snakebites with local effects. J Korean Soc Emerg Med 2004;15:523−30.
- 14. Kim ES, Choi WJ. Clinical review of venomous snake bite. J Korean Surg Soc 2000;59:433−40.
- 15. Cho NS, Park J. A clinical analysis of snake bite injury. J Korean Soc Emerg Med 1996;7:405−14.
- 16. Jang IS, Lee JA, Kim SY, et al. Clinical features in snake bite. J Korean Soc Emerg Med 1996;7:580−9.
- 17. Kim GN, Cho SW, Hwang JY. A clinical analysis on venomous snake bite in west-south area of Korea. J Korean Surg Soc 1995;48:824−31.
- 18. Fukuda T, Iwaki M, Hong SH, et al. Standardization of regional reference for mamushi (Gloydius blomhoffii) antivenom in Japan, Korea, and China. Jpn J Infect Dis 2006;59:20−4. PMID: 16495629.ArticlePubMed
- 19. Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon 2003;41:541−57. https://doi.org/10.1016/S0041-0101(02)00393-8. PMID: 10.1016/S0041-0101(02)00393-8. PMID: 12676433.ArticlePubMed
- 20. Hedeker DR, Gibbons RD. Longitudinal data analysis. Hoboken: John Wiley and Sons; 2006.
- 21. Wu JT, Zhang H. Nonparametric regression methods for longitudinal data analysis: mixed-effects modeling approaches. Hoboken: John Wiley and Sons; 2006.
- 22. Ministry of Food and Drug Safety. Korean Minimum requirements for biological products. Cheongju: Ministry of Food and Drug Safety; 2014. pp 262−4.
- 23. European Pharmacoeia Commission. Viper venom antiserum. Council of Europe; European Directorate for the Quality of Medicines & HealthCare (EDQM). European Pharmacopoeia. 7th ed. Strasbourg: Council of Europe; 2011. pp 953−4.
- 24. United States Pharmacopeial Convention. The United States Pharmacopeia; USP 34: the national formulary; NF 29. Rockville: U.S. Pharmacopeial Convention; 2011. p 1919.
- 25. Requirements for biologics of the People’s Republic of China (English edition). Beijing: Chemical Industry Press; 2000. pp 201−8.
- 26. National Institute of Infectious Diseases. Minimum requirements for biological products. Tokyo: Ministry of Health and Welfare; 2004.
- 27. Osada N, Kohara A, Yamaji T, et al. The genome landscape of the african green monkey kidney-derived vero cell line. DNA Res 2014;21:673−83. https://doi.org/10.1093/dnares/dsu029. PMID: 10.1093/dnares/dsu029. PMID: 25267831.ArticlePubMedPMC
- 28. Yasumura Y, Kawakita Y. Studies on SV40 in tissue culture-preliminary step for cancer research in vitro. Nihon Rinsho 1963;21:1201−15.
REFERENCES
Reused from Lim et al (J Korean Med Assoc 2013;56:1091–103) [7].
| Period | Patient (n) | Grade | Antivenom complication | Death | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| 0 | I | II | III | IV | Skinb | Serum sickness | ||||
| 2008–2010 | 62 | 6 | 47 | 9 | 0 | 0 | 2 | 1 | 0 | You et al (2013) [9] |
|
|
||||||||||
| 2006–2008 | 32 | 0 | 13 | 15 | 2 | 2 | – | – | 0 | Kim et al (2009) [10] |
|
|
||||||||||
| 1999–2008 | 170 | 2 | 39 | 75 | 53 | 1 | – | – | 0 | Park et al (2009) [11] |
|
|
||||||||||
| 2000–2006 | 169 | 4 | 78 | 66 | 19 | 2 | – | – | 0 | Jin et al (2008) [12] |
|
|
||||||||||
| 2000–2004 | 108 | 4 | 58 | 34 | 10 | 2 | 0 | 0 | 0 | Jun et al (2004) [13]c |
|
|
||||||||||
| 1995–1999 | 97 | 2 | 36 | 39 | 20 | 0 | – | – | 0 | Kim and Choi (2000) [14] |
|
|
||||||||||
| 1991–1996 | 71 | 15 | 25 | 26 | 5 | 0 | 3 | – | 0 | Cho and Park (1996) [15] |
|
|
||||||||||
| 1995–1996 | 100 | 3 | 47 | 37 | 13 | 0 | – | – | 0 | Jang et al (1996) [16]d |
|
|
||||||||||
| 1990–1993 | 105 | 44 | 31 | 15 | 15 | 0 | 17 | 0 | 0 | Kim et al (1995) [17] |
|
|
||||||||||
| Total | 914 | 80 | 374 | 316 | 137 | 7 | 22 | 1 | 0 | |
Modified from Lim et al (J Korean Med Assoc 2013;56:1091–103) [7].
a Traditional Snakebite Severity Grading Scale (The classification proposed by Parrish, McCollough, and Gennard).
Grade 0 (No envenomation): Local or systemic signs or symptoms absent;
Grade I (Minimal): Local swelling, absence of systemic sign, normal laboratory findings;
Grade II (Moderate): Swelling extending past bite site (6–12 inches), ≥ 1 systemic signs or symptoms, abnormal laboratory findings;
Grade III (Severe): Marked swelling (> 12 inches), tissue loss, multiple or severe systemic symptoms, immediate systemic signs, rapid progression of symptoms;
Grade IV (Very severe): Rapid development of local reaction, ecchymosis, necrosis, blebs, blisters, swelling severe enough to obstruct venous or arterial flow, swelling may involve ipsilateral trunk.
b Rash, urticarial, etc.
c Sixteen antivenom users with fever and urticaria and 1 Viken user with urticaria.
d Antivenom administrate from grade II.
Figure & Data
References
Citations

- Establishment of Reference Reagents for Single-Radial-Immunodiffusion Assay on the 2022/23 Seasonal Influenza Vaccine in Japan and Their Quality Validation
Noriko Shimasaki, Tomoko Kuwahara, Haruna Nishijima, Kazuya Nakamura, Kayoko Sato, Keiko Murano, Shigeyuki Itamura, Yukiko Akahori, Emi Takashita, Noriko Kishida, Tomoko Arita, Mina Nakauchi, Makoto Takeda, Hideki Hasegawa, Akihide Ryo, Yuichi Harada
Japanese Journal of Infectious Diseases.2024; 77(2): 105. CrossRef - A collaborative study to establish the second national standard for hepatitis B immunoglobulin in Korea
Chan Woong Choi, Su Kyoung Seong, Ki Won Han, Hyun Jeong Kim, Kyung Hee Sohn, Sun Bo Shim, Yun Su Bang, JungHwan Cho, In Soo Shin
Biologicals.2023; 82: 101679. CrossRef - Report on the seventh meeting of national control laboratories for vaccines and biologicals of the WHO Western Pacific and South-East Asia member states
Sun Bo Shim, Chan Woong Choi, Jin Ho Shin, Jong Won Kim, Silke Schepelmann, Jae Ho Jung, Harish Chander, Ratih Pujilestari, Madoka Kuramitsu, Masaki Ochiai, Nee Yuan Qi, Geraldine N. Dimapilis, Luu Thi Dung, Hyung Sil Moon, In Soo Shin
Biologicals.2023; 84: 101712. CrossRef - Effect of Agkistrodon halys antivenom in patients bit by green pit viper and the prognostic role of the disease – a retrospective cohort study
Zhong-Yi Zeng, Pei-Ying Huang, Jia-Yu Du, Yu-Xiang Liu, Shi-Gong Guo, Lin-Sheng Zeng, Cong-Cong Zhang, Yi Li
Clinical Toxicology.2022; 60(7): 808. CrossRef - Determination of the potency of a cell-based seasonal quadrivalent influenza vaccine using a purified primary liquid standard
Hitoshi Takahashi, Takao Fujimoto, Fumiaki Horikoshi, Tae Uotani, Mie Okutani, Noriko Shimasaki, Itsuki Hamamoto, Takato Odagiri, Eri Nobusawa
Biologicals.2020; 68: 32. CrossRef - The 2nd Meeting of National Control Laboratories for Vaccines and Biologicals in the Western Pacific
Hokyung Oh, Jinho Shin, Chung Keel Lee, Masaki Ochiai, Kiyoko Nojima, Chang Kweng Lim, Sanj Raut, Irene Lisovsky, Stella Williams, Ki Young Yoo, Dong-Yeop Shin, Manabu Ato, Qiang Ye, Kiwon Han, Chulhyun Lee, Naery Lee, Ji Young Hong, Kikyung Jung, Pham Va
Osong Public Health and Research Perspectives.2018; 9(3): 133. CrossRef - Measles control in Australia – threats, opportunities and future needs
C. Raina MacIntyre, Elizabeth Kpozehouen, Mohana Kunasekaran, Kathleen Harriman, Stephen Conaty, Alexander Rosewell, Julian Druce, Nicolee Martin, Anita E. Heywood, Heather F. Gidding, James Wood, Sonya Nicholl
Vaccine.2018; 36(30): 4393. CrossRef - A Collaborative Study to Establish the Second Korean National Reference Standard for Snake Venom
Kiwon Han, Kikyung Jung, Hokyung Oh, Hojin Song, Sangmi Park, Ji-Hye Kim, Garam Min, Byung-Hwa Lee, Hyun-sik Nam, Yang Jin Kim, Manabu Ato, Jayoung Jeong, Chiyoung Ahn
Toxicological Research.2018; 34(3): 191. CrossRef


 PubReader
PubReader Cite
Cite
