Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 4(5); 2013 > Article
-
Original Article
Assessment of Antioxidant Potential, Total Phenolics and Flavonoids of Different Solvent Fractions ofMonotheca Buxifolia Fruit - Shumaila Jan, Muhammad Rashid Khan, Umbreen Rashid, Jasia Bokhari
-
Osong Public Health and Research Perspectives 2013;4(5):246-254.
DOI: https://doi.org/10.1016/j.phrp.2013.09.003
Published online: October 31, 2013
Department of Biochemistry, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, Pakistan
- ∗Corresponding author. mrkhanqau@yahoo.com
© 2013 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives
- This study was conducted to investigate the antioxidant potential of methanol extract and its derived fractions (hexane, ethyl acetate, butanol, and aqueous) of fruits of Monotheca buxifolia (Falc.) Dc., a locally used fruit in Pakistan.
-
Methods
- Dried powder of the fruit of M. buxifolia was extracted with methanol and the resultant was fractionated with solvents having escalating polarity; n-hexane, chloroform, ethyl acetate, n-butanol and the residual soluble aqueous fraction. Total phenolic and total flavonoid contents were estimated for the methanol and various fractions. These fractions were also subjected to various in vitro assays to estimate the scavenging activity for 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), superoxide, hydroxyl, hydrogen peroxide and reductive ability for ferric ions and phosphomolybdate assay.
-
Results
- The n-butanol, aqueous and methanol fractions possessed high amount of phenolics and flavonoids compared with other fractions, and subsequently showed a pronounced scavenging activity on DPPH, ABTS, superoxide, hydroxyl and hydrogen peroxide radicals and had a potent reductive ability on ferric ion and phosphomolybdate assay. There was a found significant correlation between total phenolic and flavonoid contents and EC50 of DPPH, superoxide, hydrogen peroxide radical and phosphomolybdate assays, whereas a nonsignificant correlation was found with the hydroxyl radical and ABTS radical assay.
-
Conclusion
- M. buxifolia fruit can be used as natural antioxidant source to prevent damage associated with free radicals.
- The role of free radicals in many disease conditions has been well established. Oxidative stress due to the production of free radicals such as superoxide radical (O2•−), hydroxyl radical (•OH), peroxide radical (ROO•), and nitric oxide radical, is the major cause of a variety of pathological conditions including coronary heart diseases, reperfusion injury, inflammation, diabetes, drug toxicity, carcinogenesis and neurodegenerative diseases such as Parkinson and Alzheimer diseases [1].
- Antioxidant substances can block the harmful action of the free radicals by scavenging the free radicals and detoxify the organism. Synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are commonly used in food processing and preservation but have been found to have side effects and have been proved carcinogenic [2]. Thus there has been increased interest in natural antioxidants, especially those of plant origin. In the past decade there have been many reports of plant extracts and different types of phytochemicals particularly polyphenols, the secondary metabolites from plants, which were shown to have antioxidant activity [3,4]. Therefore, phenolics and other natural compounds are capable of protecting against reactive oxygen species-mediated damage with possible application in avoidance and/or curing of diseases. As safe sources of antioxidants, fruits and vegetables have been investigated for their antioxidant properties, for example blueberry (Vaccinium corymbosum L.) [5] and Launaea procumbens [6]. Fruits have been also associated conversely with aging and mortality from cardiovascular and neurodegenerative diseases [7].
- Monotheca buxifolia is a broad-leaved evergreen small tree belonging to the family Sapotaceae. This species is found in the hilly regions of Afghanistan and in Northern Pakistan. It is used as fuel, fodder, wood lumber, roof thatching materials, and particularly used as hedge around cultivated fields due to its barbed nature. This species bears small fruits, locally called Gurgura, sold in the local markets as fresh and dried food [8,9]. Medicinally, fruits have laxative and digestive properties, and are used in the treatment of urinary tract diseases. They are also used to reduce temperature in fevers and as a vermifuge [8,10,11].
- There have been no reports on the antioxidant activities of this plant previously in the literature. Hence, the present work investigates the potential antioxidant free radical scavenging effects of a methanol extract of the powder of dried fruit pulp of M. buxifolia and its derived solvent fractions, in order to understand the usefulness of this plant as a foodstuff as well as in medicinal preparations. The antioxidant capacity was evaluated for different in vitro models such as scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), hydroxyl, hydrogen peroxide (H2O2), superoxide radicals, and total antioxidant activity by the phosphomolybdate method and reducing power. Because of the effectiveness of the phenolics and flavonoids as antioxidants, the amounts of total phenolics and total flavonoids in the extracts/fractions were also determined.
Introduction
- 2.1 Chemicals
- Aluminum chloride, ascorbic acid, ferric chloride (FeCl3), ABTS, potassium persulfate, gallic acid, rutin, linoleic acid, DPPH, Folin-Ciocalteu's phenol reagent, phenazine methosulfate (PMS), nitro blue tetrazolium (NBT), trichloroacetic acid (TCA), and thiobarbituric acid (TBA) were acquired from Sigma Co. (St. Louis, MO, USA). Riboflavin, sulfuric acid, deoxyribose, sodium hydroxide (NaOH), sodium carbonate (Na2CO3), disodium hydrogen phosphate (Na2HPO4), sodium nitrite (NaNO2) and H2O2 were purchased from Wako Co. (Osaka, Japan). Ferrous chloride (FeCl2), sodium dihydrogen phosphate (NaH2PO4), potassium ferricyanide (K3Fe(CN)6), and solvents used were of analytical grade were purchased from Merck Co. (Darmstadt, Germany). Distilled deionized water (dd. H2O) was prepared using the Ultrapure water purification system (Lotus Co., Ltd., Taipei, Taiwan).
- 2.2 Plant material and extract preparation
- The fruit was collected in April 2010 from KPK province of Pakistan and the plant was identified by its local name and later identified by Dr Mir Ajab Khan, Department of Plant Sciences, Quaid-i-Azam University, Islamabad. A specimen was kept at the Herbarium of the Pakistan Museum of Natural History, Islamabad. The fruits (5.0 kg fresh) of uniform size at maturity were collected and dried under shade to obtain 1.0 kg dry sample, excluding the seeds. The dried samples were powdered in a Willy Mill to 60-mesh size and used for solvent extraction. For extract preparation, 1.0 kg of dried sample was extracted twice with 2.0 L of 95% methanol at 25 °C for 48 h. The extracts were filtered with Whatman No. 1 filter paper and evaporate to dry the filtrate by using rotary evaporator. The extract was suspended in distilled water and partitions were made with increasing polarity of solvents i.e., n-hexane, ethyl acetate, chloroform, butanol, and water. Then all fractions were dried by using rotary evaporator, and preserved at 4 °C. The dry extract was weighed and the yield was determined as the percentage of air-dried weight of plant material.
- 2.3 Estimation of total polyphenolic contents
- The total polyphenolic content was estimated using Folin-Ciocalteu reagent [12]. Folin-Ciocalteu reagent (400 μL) was mixed with 200 μL of fractions (1.0 mg/mL) in a volumetric flask. The solution was heated to 25 °C for 5–10 min and mixed with 0.2 mL of 7% Na2CO3 solution, and finally the mixture was diluted with deionized distilled water and made up to 10.0 mL in a volumetric flask. Before taking the absorbance at 725 nm, the mixture was held for 2 hours at 25 °C. A calibration curve was plotted for the standard of gallic acid. Total phenolics were calculated as equivalent of per mg gallic acid (GAE) per gram of dried sample (mg/g).
- 2.4 Estimation of total flavonoids
- Total flavonoid content was estimated according to the method of Park et al [13]. Fifty milligrams of each fraction was suspended in 10 mL of 80% methanol and filtered through Whatman filter paper No. 42 (125 mm). In a test tube (10 mL), 0.3 mL of extracts, 3.4 mL of 30% methanol, 0.15 mL of 0.5M NaNO2 and 0.15 mL of 0.3M AlCl3.6H2O was mixed. Five minutes after the addition of 1 mL of 1M NaOH, the absorbance was measured at 506 nm. Rutin was used to plot the calibration curve. Total flavonoids were calculated as mg rutin equivalents per gram of dried sample (mg/g).
- 2.5 Antioxidant activity assays
- For the antioxidant assays, all fractions were dissolved (1.0 mg/mL of fractions) in 95% methanol and a series of concentration-dependent dilutions were made. For all antioxidant assays standard chemicals were used for comparison. 2.5.1
- The DPPH assay was performed following the method of Blois [14]. DPPH (2.4 mg) was dissolved in 100 mL methanol to prepare the stock solution and then stored at 20 °C until needed. The DPPH solution was diluted with methanol to achieve an absorbance of 0.980 (±0.02) at 517 nm with the spectrophotometer. A 500-μL aliquot of the above mixture was mixed with 500 μL of the samples at different concentrations (25–250 μg/mL). The mixture was incubated in the dark for 15 minutes and the absorbance was measured at 517 nm. The DPPH scavenging activity of various fractions was calculated by the following equation:
- EC50 values calculated to determine the 50% inhibition of DPPH radicals. Ascorbic acid and rutin were used as standards. 2.5.2
- The scavenging activity assay for superoxide anion radical was performed according to the method of Beauchamp and Fridovich [15]. The reaction mixture consisted of 500 μL of 50 mM PO4 buffer (pH 7.6), 300 μL of 50 mM riboflavin, 250 μL of 20 mM phospho-methozine sulphate (PMS), and 100 μL of 0.5 mM nitroblue tetrazolium (NBT) before adding up of 1.0 mL of various fractions. Triggering of reaction was done by illuminating the above solutions using a fluorescent lamp. After 20 minutes the absorbance was measured at 560 nm. The percentage of scavenging superoxide anion generation was calculated as:
- EC50 values calculated to determine the 50% inhibition of superoxide radicals. Ascorbic acid was used as standards. 2.5.3
- The total antioxidant capacity assay of samples was carried out by the phosphomolybdenum method [16]. A 0.1-ml aliquot of the sample solution was shaken with 1 mL of reagent solution (0.6M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The test tubes were covered and incubated in a water bath at 95 °C for 90 min. After the samples were cooled, the absorbance of the mixture was measured at 765 nm. Ascorbic acid was used as standard. The antioxidant capacity was estimated using the following formula: 2.5.4
- Hydroxyl radical scavenging activity of samples was determined according to the method of Halliwell and Gutteridge [17]. The reactive mixture was consisted of: 100 μL of pre-mixed ferric chloride (100 mM), 250 μL of 2.8 mM 2-deoxyribose in 50 mM phosphate buffer (pH 7.4), and 100 mM EDTA solution (1:1; v/v); 200 mM H2O2 (50 μL) without or with the 50 μL extract solution. The reaction was triggered by adding 50 μL of ascorbate (300 mM) and heated for 60 min at 37 °C. A solution of 1% thiobarbituric acid (TBA) in 500 μL of NaOH (50 mM) and 500 μL of 2.8%TCA was added. Then the mixture was placed in a boiling water bath for 15 minutes. After cooling, the absorbance was taken at 532 nm. Hydroxyl radical scavenging was calculated as: 2.5.5
- The ABTS radical scavenging activity was determined by calculating the disappearance of the ABTS radical cation, following the method with of Re et al [18]. ABTS (7 mM) and potassium persulfate (2.4 mM) was mixed to make the stock solution and placed in the dark for 12–16 hours at room temperature. Then the solution was diluted by mixing 1 mL of ABTS+ solution with 60% methanol to obtain an absorbance of 0.708 ± 0.001 units at 734 using the spectrophotometer. For each assay ABTS+ solution was made fresh. Then 1 mL of plant extracts was added to react with 1 mL of the ABTS+ solution and the absorbance was measured at 734 nm. The decrease in absorbance was taken after 1 minute up to 6 minutes. Then the final absorbance was noted. The percentage inhibition was calculated using following formula:
- The antioxidant capacity of test samples was given by EC50, the concentration necessary for a 50% reduction of ABTS. 2.5.6
- The H2O2 radical scavenging activity of extracts was determined according to the method of Ruch et al [19]. H2O2 solution (2 mM) was prepared in 50 mM phosphate buffer (pH 7.4). An aliquot (0.1 mL) of the extract sample was placed into a test tube and the volume made up to 0.4 mL with 50 mM phosphate buffer (pH 7.4). After addition of 0.6 mL H2O2 solution, tubes were mixed and the absorbance of the H2O2 at 230 nm was determined. The ability to scavenge the H2O2 was calculated using the following equation:
- The EC50 value is the effective concentration that is required to scavenge 50% H2O2 radicals. EC50 values calculated to determine the 50% inhibition of DPPH radicals. Ascorbic acid and rutin were used as standards. 2.5.7
- The reducing power of extracts was estimated following the method of Oyaizu [20]. Extract solution (2 mL), phosphate buffer (2 mL, 0.2M, pH 6.6) and potassium ferricyanide (2 mL, 10 mg/mL) were added, and then kept at 45 °C for 30 minutes. TCA (2 mL, 100 mg/L) was added to the reaction mixture. A 2-mL aliquot of the above mixtures was added to 2 mL of distilled water and 0.4 mL of 0.1% (w/v) ferric chloride in a test tube, the absorbance was measured after 10 minutes, at 700 nm. Increased absorbance of the reaction mixture suggests a high reducing power.
- 2.6 Statistical analysis
- Readings for all antiradical scavenging assays were taken in triplicate. Graph Pad Prism 5 software (H.J. Motulsky, Prism5 Statistics Guide, GraphPad Software Inc., San Diego, CA, USA, www.Graph Pad.com) was used to calculate the EC50 values. Standard deviation and ANOVA was employed to investigate the differences among EC50 of different fractions for different antiradical assays. The Pearson correlation coefficient for phenolic and flavonoids was also employed. All the assays findings were subjected to the Student t test (p < 0.05; p < 0.01) to determine their significance.
Materials and Methods
2.5.1 DPPH assay for radical scavenging activity
2.5.2 Superoxide radical scavenging activity
2.5.3 Phosphomolybdate assay (total antioxidant capacity)
2.5.4 Hydroxyl radical scavenging activity
2.5.5 ABTS radical scavenging activity
2.5.6 H2O2-scavenging activity
2.5.7 Reducing power assay
- 3.1 Extraction yield, total phenolics and flavonoid contents
- The percentage yield, total phenolic content, and flavonoids of the methanol extract and solvent fractions obtained from M. buxifolia fruits are shown in Table 1. The recovery percentage of extractable compounds varied from 4.56 ± 1.25 to 24.18 ± 3.22. The highest yield was given by the methanol extract (24.18 ± 3.22), whereas the aqueous fraction gave the lowest (4.56 ± 1.25). Although the active components of the medicinal plants compounds are not known, polyphenols have received growing attention because of some exciting new findings concerning their biological activities. Pharmacologically, the antioxidant potential of polyphenolic compounds, particularly free radical scavenging and inhibition of lipid peroxidation, are the most important. The total phenolic compounds as recorded in Table 1 in M. buxifolia fruit fractions (determined as gallic acid equivalents or GAE), ranged between 59.13 ± 2.6 mg and 16.66 ± 1.3 mg/g dry weight of fraction. The butanol extract showed the highest total phenolics (59.13 ± 2.6 mg GAE/g fraction), whereas the phenolic contents of n-hexane were much smaller (16.66 ± 1.3 mg GAE/g), which is in agreement with other similar reports [4,21]. The antioxidant property of the compounds was well correlated with the content of their phenolic compounds [22]. Phenols contain good antioxidant, antimutagenic, and anticancer properties [23]. Flavonoids are the naturally occurring polyphenolic compounds representing one of the most prevalent classes of compounds in vegetables, nuts, fruits, and beverages such as coffee, tea, and red wine [24]. The present study showed flavonoid contents in the range of 4.11 ± 0.51 to 48.68 ± 2.8 mg as rutin equivalents/g fraction. The highest amount was observed in the aqueous fraction (48.68 ± 2.8 mg/g) followed by the methanol extract (42.045 ± 3.1 mg/g). Halliwell [25] reported that plants rich in flavonoids are potential sources of natural antioxidants that would add to the overall antioxidant capacity of an organism and inhibit lipid peroxidation. Therefore, the result suggested that phenolic acids and flavonoids may be the major contributors for the antioxidative properties and inhibitory actions toward the oxidative reaction in vitro and in vivo.
- 3.2 DPPH radical scavenging activity
- The DPPH radical has been used widely to test the antioxidant activities of plant extracts and foods. This method is based on the reduction of DPPH in methanol solution in the presence of a hydrogen-donating antioxidant, bringing about a color change from purple to yellow, which is measured at 517 nm [14]. Figure 1A shows the scavenging effect of plant fractions on DPPH radical was in the following order methanol > butanol > ethyl acetate > aqueous > n-hexane fractions. From the analysis of EC50 values (Table 2), the DPPH radical scavenging activity of the butanol fraction (24.1 ± 1.02) was found to be significantly higher (p < 0.001) followed by methanol extract (42.7 ± 1.62) and aqueous fractions (56.5 ± 3.82). The EC50 value for n-hexane fraction was found to be greater than 300 μg/mL. The EC50 value for butanol fraction was close to that of ascorbic acid (20.4 ± 0.62) and rutin (19.5 ± 1.33) used as positive controls. It shows that the M. buxifolia fruits act as antioxidants since they possess hydrogen-donating properties. The scavenging activity of the extract increased in a concentration-dependent manner.
- 3.3 Phosphomolybdate assay
- This assay is based on the reduction of phosphomolybdate ion in the presence of an antioxidant resulting in the formation of a green phosphate/MoV complex which is measured spectrophotometrically [26]. Figure 1C shows antioxidant capacity of fractions of M. buxifolia fruit in the order of aqueous > methanol > butanol > ethyl acetate > n-hexane fractions. The aqueous fraction with an EC50 value of (45.2 ± 2.53) showed high antioxidant capacity followed by the methanol fraction (56.4 ± 2.06). However, the antioxidant activity of ascorbic acid, a known antioxidant used as the positive control, was comparatively more effective than that of M. buxifolia fractions.
- 3.4 ABTS radical scavenging activity
- The ABTS assay is an excellent tool to determine the antioxidant activity of hydrogen donating and chain-breaking antioxidants. Pietta et al [27] investigated the antioxidant activity of frequently used medicinal plants and verified that the phenolic compounds are important scavengers of ABTS. Figure 1B shows that all the fractions of M. buxifolia fruit exhibited a strong scavenging activity against ABTS radicals. The butanol and aqueous fractions with respective EC50 values of (52.2 ± 2.57) and (73.1 ± 3.26) exhibited higher antioxidant activity. The butanol and aqueous extracts of M. buxifolia fruits indicate that they could cease the oxidation process by reducing free radicals. This is due to the presence of high content phenols in these extracts, as polyphenols play a vital role as antioxidants in living systems due to the presence of hydroxyl groups in ortho- and para- positions [28].
- 3.5 Hydroxyl radical scavenging activity
- The hydroxyl radical is one of the most reactive oxygen species in living systems. It damages the cell by reacting with the polyunsaturated fatty acid of cell membrane phospholipids [25]. Thus, removing OH radicals is very important for the protection of biological systems. In this study all the samples generally registered good hydroxyl radical scavenging activity in a concentration-dependent manner (25–300 μg/mL). Among them, the butanol fraction showed the highest OH scavenging potential (EC50 value of 64.1 ± 3.15). The capability of M. buxifolia fractions to eliminate hydroxyl radicals appears to directly relate to the inhibition of lipid peroxidation, and acts as scavengers of active oxygen species by breaking free radical chains.
- 3.6 H2O2 radical scavenging activity
- Our environment contains H2O2 at low concentration levels in the air, water, human body, plants, microorganisms, food, and beverages. It enters the human body through inhalation or skin contact. H2O2 is rapidly decomposed in the body into oxygen and water resulting in hydroxyl radicals (OH•) that can begin lipid peroxidation and cause damage to cell membrane and DNA. The scavenging ability of extracts of M. buxifolia fruit extracts on H2O2 radicals was in the order of aqueous > n-butanol > methanol > ethyl acetate > n-hexane fractions. Data obtained from EC50 values (Table 2) shows that the fractions showed moderate H2O2-scavenging activity. The percentage scavenging activity increased with increasing concentration of the fractions. However, the scavenging activities of ascorbic acid and rutin, used as positive controls for comparison, were relatively more evident than those of the M. buxifolia fractions.
- 3.7 Superoxide radical scavenging activity
- Figure 1D shows the superoxide radical (O2•−) scavenging activity of the samples, as measured by the riboflavin–NBT–light system in vitro. Reduction of flavins in the presence of light generates superoxide radicals which reduce NBT, forming a blue-colored formazan [15]. The fractions evaluated were found to be potent scavengers of superoxide radicals produced in the riboflavin–NBT–light system. The fractions inhibited the formation of the blue formazan in a concentration-dependent pattern and the scavenging potential was in the following order aqueous > n-butanol > ethyl acetate > methanol > n-hexane fractions. The EC50 values of aqueous and butanol fractions were 22.3 ± 1.35and 24.4 ± 0.89. These results indicated that M. buxifolia fruit fractions had a notable effect on inhibition of superoxide when compared with ascorbic acid (EC50 values 21.1 ± 0.86), which was used as positive control.
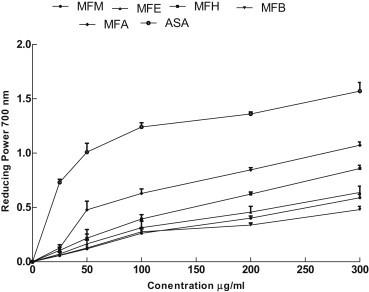
- 3.8 Reducing power activity
- Figure 2 shows the reducing power of sample extract compared to ascorbic acid the reductive capability was investigated by measuring the Fe3+–Fe2+ transformation in the presence of M. buxifolia fruit fractions, following the method of Oyaizu [20]. The reducing power of extracts is usually associated with the occurrence of reductants, which exert antioxidant action by donating a hydrogen atom and breaking the free radical chain. In our study the aqueous fraction showed highest reductive ability followed by the n-hexane fraction. The reducing power of M. buxifolia fractions suggest that it is likely to add considerably towards the overall antioxidant effect. However, the antioxidant activity of plant extracts have been recognized to have various mechanisms of action, such as binding of heavy metal ion catalysts, breakdown of peroxides, inhibition of chain initiation, reductive capacity on metals, and radical scavenging [29]. Like the antioxidant activity, the reducing power of M. buxifolia fruit fractions increased with increasing concentration of extract. However, the reducing power of ascorbic acid was comparatively more effective than that of our fractions.
- 3.9 Correlation with EC50 values of antioxidant activities and phytochemical contents
- Through correlation analysis for phytochemical contents with EC50 values of radical scavenging activities of various soluble fractions of M. buxifolia fruits, the contents of phenolics and flavonoids exhibited good correlation with phosphomolybdate assay, DPPH, superoxide, and H2O2 radical scavenging activities (Table 3). However, correlation in the case of ABTS and hydroxyl radical scavenging activity was found to be nonsignificant. The results indicate that phenolic acids and flavonoids are the major contributors to the antioxidant and free radical scavenging activities of fractions of M. buxifolia fruits, and enhanced the importance of phenolic compounds in the antioxidant property of plant extracts. The perceptible correlation among different investigations exhibited that the antioxidant assays selected in the present investigation are feasible and complementary to the antioxidant activities in natural environment. Our results are in agreement with other reports of a strong correlation of antioxidant activities and total polyphenols [4,30].
Results and Discussion
- The methanol extract of M. buxifolia fruits and its solvent fractions performed varied levels of antioxidant activity in all the in vitro models of antioxidant assays studied. The results from various free radical-scavenging systems revealed that the M. buxifolia had significant antioxidant activity and free radical-scavenging activity. The major antioxidative components appeared to be to be phenolics and flavonoids. M. buxifolia can be suggested as a potential natural source of antioxidants appropriate for utilization in nutritional/pharmaceutical fields. However, further evaluation of their bioactive compounds, antioxidant activities in living models is required.
Conclusion
-
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article information
- 1. Aruoma O.I.. Free radicals, oxidative stress and antioxidants in human health and disease. J Am Oil Chem Soc 75(2). 1998;199−212.ArticlePubMedPMC
- 2. Anagnostopoulou M.A., Kefalas P., Papageorgiou V.P.. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem 94(1). 2006 Jan;19−25.Article
- 3. Bergman M., Varshavsky L., Gottlieb H.E.. The antioxidant activity of aqueous spinach extract: chemical identification of active fractions. Phytochemistry 58(1). 2001 Sep;143−152. PMID: 11524124.ArticlePubMed
- 4. Sahreen S., Khan M.R., Khan R.A.. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem 122(4). 2010 Oct;1205−1211.Article
- 5. Castrejon A.D.R., Eichholz I., Rohn S.. Phenolic profile and antioxidant activity of high bush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem 109(3). 2008 Aug;564−572.Article
- 6. Khan R.A., Khan M.R., Sahreen S.. Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Chem Central J 6(1). 2012 May22;43. Article
- 7. Verzelloni E., Tagliazucchi D., Conte A.. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem 105(2). 2007;564−571.Article
- 8. Khan N., Ahmed M., Wahab M.. Studies along an altitudinal gradient Monotheca buxifolia forest Lower Dir District, Pakistan. Pakistan J Botany 42(5). 2010;3029−3038.
- 9. Rashid A., Marwat S.K.. Ethnobotanical study of important wild plants of Bahadur Khel Tract (Tehsil Banda Daud) in Karak District. Gomal Univ J Res 2(2). 2006;165−172.
- 10. Marwat S.K., Rehman F., Usman K.. Medico-ethnobotanical studies of edible wild fruit plants species from the flora of north western Pakistan (D. I. Khan district). J Medicinal Plants Res 5(16). 2011;3679−3686.
- 11. Ullah R., Hussain Z., Iqbal Z.. Traditional uses of medicinal plants in Darra Adam Khel, N.W.F.P., Pakistan. J Medicinal Plants Res 4(17). 2010;1815−1821.
- 12. Kim D.-O., Jeong S.W., Lee C.Y.. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry 81(3). 2003 Jun;321−326.Article
- 13. Park Y.-S., Jung S.T., Kang S.-G.. Antioxidants and proteins in ethylene-treated kiwi fruits. Food Chem 107(2). 2008 Mar;640−648.Article
- 14. Blois M.S.. Antioxidant determinations by the use of a stable free radical. Nature 181:1958;1199−1250.Article
- 15. Beauchamp C., Fridovich I.. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1). 1971 Nov;276−277. PMID: 4943714.ArticlePubMed
- 16. Umamaheswari M., Chatterjee T.K.. In vitro antioxidant activities of the fractions of Coccinnia grandis L. leaf extract. Afr J Trad Complement Altern Med 5(1). 2007 Oct;61−73.
- 17. Halliwell B., Gutteridge J.M.. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett 128(2). 1981 Jun;347−352. PMID: 6266877.ArticlePubMed
- 18. Re R., Pellegrini N., Proteggente A.. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radical Biol Med 26(9,10). 1999 May;1231−1237. PMID: 10381194.Article
- 19. Ruch R.J., Cheng S.-J., Klaunig J.E.. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10(6). 1989 Jun;1003−1008. PMID: 2470525.ArticlePubMed
- 20. Oyaizu M.. Antioxidant activity of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography. Nippon Shokulin Kogyo Gakkaishi 35(11). 1988 Nov;771−775.Article
- 21. Ao C., Li A., Elzaawely A.A.. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fill extract. Food Control 19(10). 2008 Oct;940−948.Article
- 22. Velioglu Y.S., Mazza G., Gao L.. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 46(10). 1998 Jan;4113−4117.Article
- 23. Ahmad N., Mukhtar H.. Green tea polyphenols and cancer: biological mechanisms and practical implications. Nutr Rev 57(3). 1999 Mar;78−83. PMID: 10101921.ArticlePubMed
- 24. Hertog M.G.L., Hollman P.C.H., Van de Putte B.. Content of potentially anticarcinogenic flavonoids of tea infusion wines and fruit juices. J Agric Food Chem 41(8). 1993 Aug;1242−1246.Article
- 25. Halliwell B.. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 476(2). 2008 Aug15;107−112. PMID: 18284912.ArticlePubMed
- 26. Prieto P., Pineda M., Aguilar M.. Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application of vitamin E. Anal Biochem 269(2). 1999 May;337−341. PMID: 10222007.ArticlePubMed
- 27. Pietta P., Simonetti P., Mauri P.. Antioxidant activity of selected medicinal plants. J Agric Food Chem 46(11). 1998 Oct;4487−4490.Article
- 28. Lapornik B., Prosek M., Wondra G.A.. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J Food Eng 71(2). 2005 Nov;214−222.Article
- 29. Diplock A.T.. Will the good fairies please prove us that vitamin E lessens human degenerative disease? Free Radical Res 27(5). 1997 Nov;511−532. PMID: 9518068.
- 30. Khan R.A., Khan M.R., Sahreen S.. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem Central J 6(1). 2012 Feb 6;12. Article
References

|
Correlation R² |
||
|---|---|---|
| EC50 | Phenolics | Flavonoids |
| EC50 of scavenging ability on DPPH radicals | 0.9295∗ | 0.8554∗ |
| EC50 of scavenging ability on superoxide | 0.7947† | 0.9202∗ |
| EC50 of phosphomolybdate assay | 0.7732† | 0.9648∗ |
| EC50 of scavenging ability on hydroxyl radicals | 0.1661 | 0.004 |
| EC50 of scavenging ability on hydrogen peroxide radicals | 0.6779† | 0.8995∗ |
| EC50 of scavenging ability on ABTS radicals | 0.3992 | 0.2406 |
Figure & Data
References
Citations

- Global omics study of Tetraselmis chuii reveals time-related metabolic adaptations upon oxidative stress
Aikaterini Koletti, Dimitrios Skliros, Chrysanthi Kalloniati, Sofia Marka, Maria-Eleftheria Zografaki, Carlos Infante, Lalia Mantecón, Emmanouil Flemetakis
Applied Microbiology and Biotechnology.2024;[Epub] CrossRef - Multivariate Sonication-Based Extraction Optimization to Improve the Antioxidant and Anti-Inflammatory Properties of Anacyclus pyrethrum var. Pyrethrum Root Extracts
Oumaima Chater, Smail Aazza, Helena Silva, Ahmed Harrach, Lahsen El Ghadraoui
Chemistry Africa.2024;[Epub] CrossRef - Optimization of protein extraction from sesame seeds by using response surface methodology RSM
Roua Khalfallah, Manel Mechmeche, Hamida Ksontini, Ines Jmoui, Moktar Hamdi, Faten Kachouri
Nutrire.2024;[Epub] CrossRef - Tea (Camellia sinensis) cultivated in three agro-ecological regions of Bangladesh: Unveiling the variability of methylxanthine, bioactive phenolic compound, and antioxidant activity
Abu Tareq Mohammad Abdullah, Mahbuba Ibrahim Sayka, Mohammad Mahfuzur Rahman, Miskat Sharif, Tanzir Ahmed Khan, Sharmin Jahan, Reaz Mohammad Mazumdar, Mohammad Nashir Uddin, Md. Mozammel Hoque
Heliyon.2024; 10(7): e28760. CrossRef - Synthesis and characterization of marine seagrass (Cymodocea serrulata) mediated titanium dioxide nanoparticles for antibacterial, antibiofilm and antioxidant properties
Mohankumar Narayanan, Suganthi Srinivasan, Chackaravarthi Gnanasekaran, Govindan Ramachandran, Chenthis Kanisha Chelliah, Govindan Rajivgandhi, Muthuchamy Maruthupandy, Franck Quero, Wen-Jun Li, Gasim Hayder, Jamal M. Khaled, Arulraj Arunachalam, Natesan
Microbial Pathogenesis.2024; 189: 106595. CrossRef - Oroxylum indicum Stem Bark Extract Reduces Tumor Progression by Inhibiting the EGFR-PI3K-AKT Pathway in an In Vivo 4NQO-Induced Oral Cancer Model
Munia Parvin, Ashikur Rahaman, Arnab Sarkar, Sudhan Debnath, Utpal Chandra De, Deba Prasad Mandal, Shamee Bhattacharjee
Journal of the American Nutrition Association.2023; 42(6): 573. CrossRef - Alternanthera brasiliana L. extract alleviates carbon tetrachloride-induced liver injury and fibrotic changes in mice: Role of matrix metalloproteinases and TGF-β/Smad axis
Vinay M. Paliwal, Sourav Kundu, Uttam Kulhari, Aishwarya Jala, Sharmeen Ishteyaque, Roshan M. Borkar, Madhav Nilakanth Mugale, Upadhyayula Suryanarayana Murty, Bidya Dhar Sahu
Journal of Ethnopharmacology.2023; 303: 115992. CrossRef - Optimization of Extraction Parameters and Characterization of Tunisian Date Extract: A Scientific Approach Toward Their Utilization
Nesrine Messadi, Manel Mechmeche, Khaoula Setti, Zoulikha Tizemmour, Moktar Hamdi, Faten Kachouri
Sugar Tech.2023; 25(2): 460. CrossRef - An overview of the effect of medicinal herbs on pain
Namdar Yousofvand, Boshra Moloodi
Phytotherapy Research.2023; 37(3): 1057. CrossRef - Mass spectrometry-based metabolomics approach and in vitro assays revealed promising role of 2,3-dihydroquinazolin-4(1H)-one derivatives against colorectal cancer cell lines
Lina A. Dahabiyeh, Farah Hudaib, Wafa Hourani, Wesam Darwish, Bashaer Abu-Irmaileh, Pran Kishore Deb, Katharigatta N. Venugopala, Viresh Mohanlall, Sandeep Chandrashekharappa, Rana Abu-Dahab, Mohammad H. Semreen, Yasser Bustanji
European Journal of Pharmaceutical Sciences.2023; 182: 106378. CrossRef - Phenolic Compounds Profiling and Their Antioxidant Capacity in the Peel, Pulp, and Seed of Australian Grown Avocado
Xiaoyan Lyu, Osman Tuncay Agar, Colin J. Barrow, Frank R. Dunshea, Hafiz A. R. Suleria
Antioxidants.2023; 12(1): 185. CrossRef - Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature
Shaima Al Hasani, Zahir Al-Attabi, Mostafa Waly, Nasser Al-Habsi, Lyutha Al-Subhi, Mohammad Shafiur Rahman
Foods.2023; 12(4): 871. CrossRef -
Analysis of
Piper betle
L. Leaves from Bangladesh for Polyphenolics by Ultrasonic-Assisted Extraction (UAE) and High-Performance Liquid Chromatography (HPLC) Together with the Antioxidant, Antibacterial, and Cytotoxic
Md Atikul Islam, Ji Young Jeong, Md Selim Hossain, Hasan Tarek, Naeem Khan, Nargis Jamila, Kyong Su Kim
Analytical Letters.2023; 56(18): 2851. CrossRef - Nutritional Value, Phytochemical Composition, and Antioxidant Activities of Phoenix dactylifera L.: Comparison Between Fleshes and Seeds of Tunisian and Algerian Varieties
Nesrine Messadi, Manel Mechmeche, Khaoula Setti, Zoulikha Tizemmour, Moktar Hamdi, Faten Kachouri
Chemistry Africa.2023; 6(5): 2471. CrossRef - Antioxidant, Anti‐Skin‐Aging, Anti‐Inflammatory, and Anti‐Acetylcholinesterase Activities of Rourea oligophlebia Extracts
Visarut Buranasudja, Sornkanok Vimolmangkang, Kittipong Sanookpan, Asma Binalee, Anh Bao Nguyen, Ut Dong Thach, Bich Hang Do, Van Son Dang, Kiep Minh Do, Hien Minh Nguyen
Chemistry & Biodiversity.2023;[Epub] CrossRef - New Records of Tetraselmis sp. Strains with Biotechnological Potential Isolated from Greek Coastal Lagoons
Alexandros Ntzouvaras, Xanthi Chantzistrountsiou, Niki Papageorgiou, Aikaterini Koletti, Ioannis-Dimosthenis Adamakis, Maria-Eleftheria Zografaki, Sofia Marka, Gabriel Vasilakis, Amerssa Tsirigoti, Ioannis Tzovenis, Emmanouil Flemetakis, Athena Economou-A
Water.2023; 15(9): 1698. CrossRef - Functional insight of siderophore in reducing cadmium stress and inducing growth promotion in Solanum melongena
Gaurav Yadav, Neha Sharma, Surbhi Dabral, Anukool Vaishnav, Ajit Varma, S.L. Kothari, D.K. Choudhary
South African Journal of Botany.2023; 158: 479. CrossRef - Exploring the therapeutic potential of Decalepis hamiltonii root extract: synthesis of gold nanoparticles and assessment of antimicrobial, antioxidant, and anti-proliferative activities
Ekambaram Gayathiri, Palanisamy Prakash, Kuppusamy Selvam, Thangaraj Pradeep, Ravishankar Ram Mani, Sumathi Jones, Deepa Kandaswamy, Daoud Ali, Saud Alarifi, Soon Woong Chang, Balasubramani Ravindran
Applied Nanoscience.2023; 13(9): 5967. CrossRef - Secondary metabolite profiling, antioxidant capacity, enzyme inhibitory potential and in silico studies of Launaea intybacea (Jacq.) Beauverd: A multifunctional approach to probe into the new nutraceuticals
Qurat-ul-Ain, Muhammad Saleem, Mamona Nazir, Naheed Riaz, Muhammad Imran Tousif, Saba Tauseef, Laiba Hassan, Gokhan Zengin, Majid Sharifi-Rad, Syed Adnan Ali Shah
Journal of Molecular Structure.2023; 1294: 136480. CrossRef - Modulation of digestibility of canine food using enzyme supplement: an in vitro simulated semi-dynamic digestion study
Swati Jadhav, Tejal Gaonkar, Mithila Joshi, Abhijit Rathi
Frontiers in Veterinary Science.2023;[Epub] CrossRef - Assessment of Chemical Constituents of Allium sativum Essential Oil Extracted by using Hydrodistillation Technique and their Pharmacological Potential
Kusum Sharma, Veena Sharma
Journal of Natural Remedies.2023; : 977. CrossRef - Bioactivity profiling of native and hybrid varieties of pumpkin peel (Cucurbita maxima Linn.)
Tasmina Ferdous Susmi, Moshiur Rahman Khan, Nahid Hasan, Asmim Aktar, M. Ziaul Amin
Journal of Agriculture and Food Research.2023; 14: 100813. CrossRef - Exploring the therapeutic potential of edible Pleurotus mushroom species for oxidative stress and diabetes management
Karempudi Venkata Krishna, Jimmantiyur Madhappan Murugan, Haroon Khan, Munusamy Kumar, Veeramani Veeramanikandan, Ashraf Atef Hatamleh, Munirah Abdullah Al-Dosary, Karthikeyan Venkatachalam, Paulraj Balaji
Journal of King Saud University - Science.2023; 35(9): 102926. CrossRef - Synthesis and Characterization of Cosmetic Grade Ionic Liquids and Their Application as Skin Whitening Agents
Farah Bashir, Nawshad Muhammad, Meshal Alshamrani, Maliha Uroos, Ahmad Salawi, Awaji Y. Safhi, Naveed Ahmed, Abdullah Alsalhi, Fahad Y. Sabei, Osama A. Madkhali
Journal of Pharmaceutical Innovation.2023; 18(4): 2277. CrossRef - Flavonoid-rich fraction of Lasianthera africana leaves alleviates hepatotoxicity induced by carbon tetrachloride in Wistar rats
Daniel Emmanuel Ekpo, Parker Elijah Joshua, Arome Solomon Odiba, Okwesilieze Fred Chiletugo Nwodo
Drug and Chemical Toxicology.2022; 45(5): 1934. CrossRef - Algerian Sonchus oleraceus L.: a comparison of different extraction solvent on phytochemical composition, antioxidant properties and anti-cholinesterase activity
Fatine Aissani, Nedjoud Grara, Chawki Bensouici, Aissam Bousbia, Hayette Ayed, Muhd Hanis Md Idris, Lay Kek Teh
Advances in Traditional Medicine.2022; 22(2): 383. CrossRef - Antioxidative-, Antimicrobial-, Enzyme Inhibition-, and Cytotoxicity-Based Fractionation and Isolation of Active Components from Monotheca buxifolia (Falc.) A. DC. Stem Extracts
Joham Sarfraz Ali, Naheed Riaz, Abdul Mannan, Saira Tabassum, Muhammad Zia
ACS Omega.2022; 7(4): 3407. CrossRef - Antioxidant potential, anti-nutritional factors, volatile compounds and phenolic composition of microwave heat-treated plum (Prunus domestica. L.) kernels: an analytical approach
Mohd Aaqib Sheikh, Charanjiv Singh Saini, Harish Kumar Sharma
British Food Journal.2022; 124(10): 3236. CrossRef - Antimicrobial, anti-inflammatory and antioxidant activities of natural organic matter extracted from cretaceous shales in district Nowshera-Pakistan
Fazli Khuda, Madiha Anjum, Suleman Khan, Hamayun Khan, Muhammad Umar Khayam Sahibzada, Ameer Khusro, Asif Jan, Naveed Ullah, Yasar Shah, Zakiullah, Muhammad Abbas, Tayyaba Iftikhar, Abubakr M. Idris, Mayeen Uddin Khandaker, Talha Bin Emran
Arabian Journal of Chemistry.2022; 15(2): 103633. CrossRef - Combined effect of microwave and hydrothermal treatment on anti-nutritional factors, antioxidant potential and bioactive compounds of plum (Prunus domestica L.) kernels
Mohd Aaqib Sheikh, Charanjiv Singh Saini
Food Bioscience.2022; 46: 101467. CrossRef - Identification of Phenolic Compounds in Australian-Grown Bell Peppers by Liquid Chromatography Coupled with Electrospray Ionization-Quadrupole-Time-of-Flight-Mass Spectrometry and Estimation of Their Antioxidant Potential
Zexing Leng, Biming Zhong, Hanjing Wu, Ziyao Liu, Abdur Rauf, Sami Bawazeer, Hafiz Ansar Rasul Suleria
ACS Omega.2022; 7(5): 4563. CrossRef - Protective effect of Gymnema sylvestre leaf extract against uranium toxicity in human peripheral blood mononuclear cells
Sherin John Joseph, Shanmugapriya Shanmugasundaram, Mohammed Junaid Hussain Dowlath, Kantha Deivi Arunachalam, P. Balakrishna Murthy, Avinash Ashok Kadam, R. Rajakrishnan, Rengasamy Sathya, Sasikala Chinnappan
Journal of King Saud University - Science.2022; 34(3): 101895. CrossRef - Genetic and seasonal variability of bioactive polyphenolic compounds and antioxidant‐based phytonutrient promise of diverse vegetable amaranths of Indo‐Gangetic plains of West Bengal
Debasmita Sen, Soumen Bhattacharjee
JSFA reports.2022; 2(3): 116. CrossRef - LC-ESI-QTOF-MS/MS Characterization and Estimation of the Antioxidant Potential of Phenolic Compounds from Different Parts of the Lotus (Nelumbo nucifera) Seed and Rhizome
Zihan Zhu, Biming Zhong, Zihong Yang, Wanrong Zhao, Linghong Shi, Ahsan Aziz, Abdur Rauf, Abdullah S.M. Aljohani, Fahad A. Alhumaydhi, Hafiz Ansar Rasul Suleria
ACS Omega.2022; 7(17): 14630. CrossRef - Quality Control, Anti-Hyperglycemic, and Anti-Inflammatory Assessment of Colvillea racemosa Leaves Using In Vitro, In Vivo Investigations and Its Correlation with the Phytoconstituents Identified via LC-QTOF-MS and MS/MS
Mohamed S. Abd El Hafeez, Omayma El Gindi, Mona H. Hetta, Hanan F. Aly, Safwat A. Ahmed
Plants.2022; 11(6): 830. CrossRef - The Synergetic Effect of Egyptian Portulaca oleracea L. (Purslane) and Cichorium intybus L. (Chicory) Extracts against Glucocorticoid-Induced Testicular Toxicity in Rats through Attenuation of Oxidative Reactions and Autophagy
Samar R. Saleh, Ashraf Manaa, Eman Sheta, Doaa A. Ghareeb, Nihad M. Abd-Elmonem
Antioxidants.2022; 11(7): 1272. CrossRef - Phytochemistry, biological activities and in silico molecular docking studies of Oxalis pes-caprae L. compounds against SARS-CoV-2
Farhat Gul, Ilham Khan, Javed Iqbal, Banzeer Ahsan Abbasi, Amir Shahbaz, Raffaele Capasso, Itzel Amaro-Estrada, Yousef A. Bin Jardan, Raquel Cossio-Bayugar, Tariq Mahmood
Journal of King Saud University - Science.2022; 34(6): 102136. CrossRef - Exploring the industrial importance of a miracle herb Withania somnifera (L.) Dunal: Authentication through chemical profiling, in vitro studies and computational analyses
Muhammad Imran Tousif, Mamona Nazir, Muhammad Saleem, Saba Tauseef, Reaz Uddin, Muhammad Altaf, Gokhan Zengin, Gunes Ak, Refiye Beyza Ozturk, Mohamad Fawzi Mahomoodally
Process Biochemistry.2022; 121: 514. CrossRef - New mechanistic insights on Justicia vahlii Roth: UPLC-Q-TOF-MS and GC–MS based metabolomics, in-vivo, in-silico toxicological, antioxidant based anti-inflammatory and enzyme inhibition evaluation
Abdul Basit, Saeed Ahmad, Kashif ur Rehman Khan, Asmaa E. Sherif, Hanan Y. Aati, Chitchamai Ovatlarnporn, Mohsin Abbas Khan, Huma Rao, Imtiaz Ahmad, Muhammad Nadeem Shahzad, Bilal Ahmad Ghalloo, Hassan Shah, Kifayat Ullah Khan, Rizwana Dilshad
Arabian Journal of Chemistry.2022; 15(10): 104135. CrossRef - New insight into the pigmented rice of northeast India revealed high antioxidant and mineral compositions for better human health
Sagolshem Priyokumar Singh, Vanlalsanga, S.K. Mehta, Y. Tunginba Singh
Heliyon.2022; 8(8): e10464. CrossRef - Comparative phytochemical screening through high-performance thin layer chromatography technique and free radical scavenging ability of five species of genus Clerodendrum
Himangshu Sarma, Deepak Rabha, Puspanjali Khound, Nonibala Gurumayum, Partha Pratim Sarma, Partha Pratim Dutta, Paramita Choudhury, Kangkon Saikia, Sumi Pait, Jagat Chandra Borah, Dharmeswar Barman, Arundhuti Devi, Dulal Chandra Boruah, Rajlakshmi Devi
Vegetos.2022; 36(3): 1013. CrossRef - Antioxidant, Antimicrobial, and Kinetic Studies of Β-Cyclodextrin Crosslinked with Lignin for Drug Delivery
Narcis Anghel, Violeta Melinte, Iuliana Spiridon, Mihaela Pertea
Pharmaceutics.2022; 14(11): 2260. CrossRef - Relative evaluation of in-vitro antioxidant potential and phenolic constituents by HPLC-DAD of Brassica vegetables extracted in different solvents
Mohammad Mahfuzur Rahman, Abu Tareq Mohammad Abdullah, Miskat Sharif, Sharmin Jahan, Md. Alamgir Kabir, Md. Motalab, Tanzir Ahmed Khan
Heliyon.2022; 8(10): e10838. CrossRef - Bioactive polyphenolic compounds, water-soluble vitamins, in vitro anti-inflammatory, anti-diabetic and free radical scavenging properties of underutilized alternate crop Amaranthus spinosus L. from Gangetic plain of West Bengal
Agnideepa Kar, Soumen Bhattacharjee
Food Bioscience.2022; 50: 102072. CrossRef - Green Synthesis of Zinc Oxide Nanoparticles Using Monotheca buxifolia Leaf Extract; Their Biological Activities and Use in Fabrication of Nano-Biosensor
M. Zahoor, S. Naz, S. Amin, M. Iftikhar, N. Nazir, A. W. Kamran, F. A. Khan
Surface Engineering and Applied Electrochemistry.2022; 58(5): 555. CrossRef - Chemical composition, antibacterial efficacy, and antioxidant capacity of essential oil and oleoresin from Monodora myristica and Tetrapleura tetraptera in Southeast Nigeria
Queency N. Okechukwu, Fabian U. Ugwuona, Chigozie E. Ofoedu, Szymon Juchniewicz, Charles Odilichukwu R. Okpala
Scientific Reports.2022;[Epub] CrossRef - Fresh Pod Yield, Physical and Nutritional Quality Attributes of Common Bean as Influenced by Conventional or Organic Farming Practices
Ioannis Karavidas, Georgia Ntatsi, Sofia Marka, Theodora Ntanasi, Beppe Consentino, Leo Sabatino, Pietro Iannetta, Dimitrios Savvas
Plants.2022; 12(1): 32. CrossRef - Relative Evaluation of In-Vitro Antioxidant Potential and Phenolic Constituents by HPLC-DAD of Brassica Vegetables Extracted in Different Solvents
Mohammad Mahfuzur Rahman, Abu Tareq Mohammad Abdullah, Miskat Sharif, Sharmin Jahan, Md. Alamgir Kabir, Md. Motalab, Tanzir Ahmed Khan
SSRN Electronic Journal .2022;[Epub] CrossRef - In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions
Magdalene M. Aondona, Julius K. Ikya, Moses T. Ukeyima, Tsav‐wua J. A. Gborigo, Rotimi E. Aluko, Abraham T. Girgih
Journal of Food Biochemistry.2021;[Epub] CrossRef - Integrating of Aqueous Enzymolysis with Ethanolic Ultrasonication to Ameliorate Cordycepin Content from Vietnamese Cordyceps militaris and Biological Analysis of Extracts
Vuong Hoai Thanh, Phan Le Thao My, Tran Do Dat, Nguyen Duc Viet, Nguyen Cao Minh Phuc, Ngo Hong Thao, Hoang Minh Nam, Mai Thanh Phong, Nguyen Huu Hieu
ChemistrySelect.2021; 6(1): 16. CrossRef - WITHDRAWN: The free radicals scavenging potential of methanol extract of seeds (Horse gram) of Macrotyloma uniflorum
Manikandan Mathaiyan, Suriyavathana Muthukrishnan, Anandhi Eswaran, Kavitha Rani Mari, Punithavathi Manogaran
Materials Today: Proceedings.2021;[Epub] CrossRef - Comparison of methanolic extracts of Doronicum orientale and Echium angustifolium in terms of chemical composition and antioxidant activities
Radjassegarin Arumugam, Cengiz Sarikurkcu, Mehmet Sabih Ozer
Biocatalysis and Agricultural Biotechnology.2021; 33: 101984. CrossRef - Identification of phenolic compounds in Australian grown dragon fruits by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential
Zhicong Chen, Biming Zhong, Colin J. Barrow, Frank R. Dunshea, Hafiz A.R. Suleria
Arabian Journal of Chemistry.2021; 14(6): 103151. CrossRef - Polyphenolics and therapeutic insights in different tissues extract and fractions of Camellia sinensis (L.) Kuntze (Kangra Tea)
Ranjana Sharma, Sarika Verma, Dinesh Kumar
Food Bioscience.2021; 42: 101164. CrossRef - Assessment of Radical Scavenging Activity and Estimation of EC50 Values of Various Extracts of Leaves and Roots from Lobelia nicotianifolia Roth. (Wild Tobacco)
Rupali M. Kolap, Prachi S. Kakade, Rajesh N Gacche, Saurabha B. Zimare
Journal of Herbs, Spices & Medicinal Plants.2021; 27(4): 343. CrossRef - Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax senticosus and Their Enrichment and Antioxidant Properties
Ruixue Liu, Xiuling Chu, Jianqing Su, Xiang Fu, Qibin Kan, Xiaoya Wang, Xinyu Zhang
Processes.2021; 9(10): 1708. CrossRef - Physiological Roles of Red Carrot Methanolic Extract and Vitamin E to Abrogate Cadmium-Induced Oxidative Challenge and Apoptosis in Rat Testes: Involvement of the Bax/Bcl-2 Ratio
Ahmed Abdel-Wahab, Kamel M. A. Hassanin, Ahmed A. Mahmoud, Walaa I. E. Abdel-Badeea, Abdel-Razik H. Abdel-Razik, Eman Zekry Attia, Usama Ramadan Abdelmohsen, Rabie L. Abdel Aziz, Agnieszka Najda, Ibtesam S. Alanazi, Khalaf F. Alsharif, Mohamed M. Abdel-Da
Antioxidants.2021; 10(11): 1653. CrossRef - Phytochemical analysis and in vitro Antioxidant and Antibacterial activity of the Chloroform leaf extract of Abelmoschus manihot (L.) Medik (Malvaceae)
Selvaraj Divya, Subramanian Arivoli, Samuel Tennyson
Research Journal of Pharmacy and Technology.2021; : 4719. CrossRef - Total Phenolics, Flavonoids and in vitro Antioxidant Properties of Lophira lanceolata TIEGH
Collins Azubuike O, Martha Nneoma Ofo, Matthias Onyebuchi
Research Journal of Medicinal Plants.2021; 15(1): 29. CrossRef - Chemical composition, antioxidant and enzyme inhibitory properties of Ajuga parviflora Benth.
Amrita Suryavanshi, Suresh Kumar, Dolly Kain, Atul Arya
Biocatalysis and Agricultural Biotechnology.2021; 37: 102191. CrossRef - Polyphenols content of selected medical plants and food supplements present at Bulgarian market
Petya Koleva, Silvia Tsanova-Savova, Slaveyka Paneva, Stefan Velikov, Zaharina Savova
Pharmacia.2021; 68(4): 819. CrossRef - Investigation on the Phytochemical Composition, Antioxidant and Enzyme Inhibition Potential of Polygonum Plebeium R.Br: A Comprehensive Approach to Disclose New Nutraceutical and Functional Food Ingredients
Muhammad Saleem, Natasha Shazmeen, Mamona Nazir, Naheed Riaz, Gokhan Zengin, Hafiz Muhammad Ataullah, Qurat‐ul‐Ain, Farrukh Nisar, Mahreen Mukhtar, Muhammad Imran Tousif
Chemistry & Biodiversity.2021;[Epub] CrossRef - PHYTOCHEMICAL SCREENING AND CHARACTERIZATION OF VOLATILE COMPOUNDS BY GAS CHROMATOGRAPHY-MASS SPECTROMETRY FROM “NEPHROLEPIS EXALTATA”
DEEPAK KUMAR SHARMA, DAVE RS, SHAH KR
Asian Journal of Pharmaceutical and Clinical Resea.2021; : 93. CrossRef - Antimicrobial, cytotoxic, phytochemical and biological properties of crude extract and solid phase fractions of Monotheca buxifolia
Joham Sarfraz Ali, Ihsan Khan, Muhammad Zia
Advances in Traditional Medicine.2020; 20(1): 115. CrossRef - Farklı Solvent Tipi ile Yapılan Ekstraksiyon İşleminin Hünnap (Ziziphus jujube) Meyvesinin Biyoaktif Özellikleri Üzerine Etkisi
Beyza ÇİFTÇİ, Kevser KARAMAN, Mahmut KAPLAN
Türk Tarım ve Doğa Bilimleri Dergisi.2020; 7(1): 256. CrossRef - A comparative study on the role of Omani honey with various food supplements on diabetes and wound healing
Hemadri Reddy Salla, Fatma Sabeeah Al Habsi, Heba Mohammed Al dholi, Saleema Tahani Al musallami, Warda Hamed Al Sharji
Journal of King Saud University - Science.2020; 32(3): 2122. CrossRef - Evaluation of antioxidant and anti-inflammatory potency of Lepidium pinnatifidum Ledeb
Saira Bibi, Munazza Anwar, Huma Farooque Hashmi, Muhammad Rashid Khan
Clinical Phytoscience.2020;[Epub] CrossRef - Biological and Phytochemicals Properties of Monotheca buxifolia: An Unexplored Medicinal Plant
Ihsan Khan, Joham Sarfraz Ali, Ihsan Ul-Haq, Muhammad Zia
Pharmaceutical Chemistry Journal.2020; 54(3): 293. CrossRef - Bioactive polyphenols from Ranunculus macrophyllus Desf. Roots: Quantification, identification and antioxidant activity
Amirouche Deghima, Nadjat Righi, Noelia Rosales-Conrado, María Eugenia León-González, Esther Gómez-Mejía, Yolanda Madrid, Faiza Baali, Fatiha Bedjou
South African Journal of Botany.2020; 132: 204. CrossRef - Metabolic fingerprinting, antioxidant characterization, and enzyme-inhibitory response of Monotheca buxifolia (Falc.) A. DC. extracts
Joham Sarfraz Ali, Hammad Saleem, Abdul Mannan, Gokhan Zengin, Mohamad Fawzi Mahomoodally, Marcello Locatelli, Syafiq Asnawi Zainal Abidin, Nafees Ahemad, Muhammad Zia
BMC Complementary Medicine and Therapies.2020;[Epub] CrossRef - Effect of Heavy Metals on the Andrographolide Content, Phytochemicals and Antioxidant Activity of Andrographis paniculata
Anna Antony, Praveen Nagella
Asian Journal of Chemistry.2020; 32(11): 2748. CrossRef - Comparative Polyphenol Composition, Antioxidant and Anticorrosion Properties in Various Parts of Panax ginseng Extracted in Different Solvents
Ramalingam Malathy, Mayakrishnan Prabakaran, Kathirvel Kalaiselvi, Ill-Min Chung, Seung-Hyun Kim
Applied Sciences.2020; 11(1): 93. CrossRef - Bioactivity-guided isolation, characterization, and estimation of esculetin – A Potential Marker from Launaea pinnatifida cass
Himanshu Makwana, Devang Pandya
Pharmacognosy Magazine.2020; 16(72): 713. CrossRef - Total Phenolic and Flavonoid Content and In Vitro Antioxidant Activity of Methanol Extract and Solvent Fractions of Desmodium ramosissimum G. Don
Uchechukwu Ezealigo, Parker Joshua, Chidinma Ononiwu, Matthias Agbo, Rita Asomadu, Victor Ogugua
Medical Sciences Forum.2020; 2(1): 15. CrossRef - Evaluation of Antioxidant and Anticorrosion Properties of Epipremnum aureum
Mayakrishnan Prabakaran, Venkatesan Hemapriya, Seung-Hyun Kim, Ill-Min Chung
Arabian Journal for Science and Engineering.2019; 44(1): 169. CrossRef - Shelf-life and quality attributes in fresh-cut pear cv. Shahmive treated with different kinds of antioxidants
Elahe Akbari, Mahdiyeh Gholami, Cyrus Ghobadi
Journal of Food Science and Technology.2019; 56(9): 3998. CrossRef - Phytochemical characteristics of callus suspension culture of Helicteres angustifolia L. and its in vitro antioxidant, antidiabetic and immunomodulatory activities
X. Yang, Z. Lei, Y. Yu, L. Xiao, D. Cheng, Z. Zhang
South African Journal of Botany.2019; 121: 178. CrossRef - Monotheca buxifolia mediated synthesis of gold nanoparticles and its diverse biomedical applications
Asma Shah, Kafeel Ahmad, Ali Talha Khalil, Farhat Amin, Ghosia Lutfullah, Khalid Khan, Ghazala Shah, Aftab Ahmad
Materials Research Express.2019; 6(12): 125416. CrossRef - Evaluation of Polyphenolics Content and Antioxidant Activity in Edible Wild Fruits
Sharui Shan, Xuming Huang, Munir H. Shah, Arshad Mehmood Abbasi
BioMed Research International.2019; 2019: 1. CrossRef - Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus (Schott & Endl.) R.Br.
Riffat Batool, Muhammad Rashid Khan, Moniba Sajid, Saima Ali, Zartash Zahra
BMC Chemistry.2019;[Epub] CrossRef - Effects of ripening stage on the content and antioxidant capacities of phenolic compounds of arils, seeds and husks of ackee fruit Blighia sapida Köenig
Alex Sybron, Dilip K. Rai, Kisan R. Vaidya, Mohammad B. Hossain, Noureddine Benkeblia
Scientia Horticulturae.2019; 256: 108632. CrossRef - Study on novel antibacterial and antiviral compounds from abalone as an important marine mollusc
Peter Valtchev
Journal of Aquaculture & Marine Biology.2018;[Epub] CrossRef - Evaluation of Antioxidant Properties, Phenolic Compounds, Anthelmintic, and Cytotoxic Activities of Various Extracts Isolated from Nepeta cadmea: An Endemic Plant for Turkey
Arzu Kaska, Nahide Deniz, Mehmet Çiçek, Ramazan Mammadov
Journal of Food Science.2018; 83(6): 1552. CrossRef - Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera
Mayakrishnan Prabakaran, Seung-Hyun Kim, Asokan Sasireka, Murugesan Chandrasekaran, Ill-Min Chung
Food Bioscience.2018; 26: 23. CrossRef - Evaluation of in vivo antioxidant potential of Syzygium jambos (L.) Alston and Terminalia citrina Roxb. towards oxidative stress response in Saccharomyces cerevisiae
Jobina Rajkumari, Madhu Dyavaiah, S. J. Sudharshan, Siddhardha Busi
Journal of Food Science and Technology.2018; 55(11): 4432. CrossRef - Design of biosystems to provide healthy and safe food. Part A: effect of emulsifier and preparation technique on physicochemical, antioxidant and antimicrobial properties
Behnoush Maherani, Mohamed Ali Khlifi, Stephane Salmieri, Monique Lacroix
European Food Research and Technology.2018; 244(11): 1963. CrossRef - Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents
Nisha Thavamoney, Leykkha Sivanadian, Lee Hong Tee, Hock Eng Khoo, Krishnamurthy Nagendra Prasad, Kin Weng Kong
Journal of Food Science and Technology.2018; 55(7): 2523. CrossRef - Production of antioxidant peptide fractions from a by-product of tomato processing: mass spectrometry identification of peptides and stability to gastrointestinal digestion
Nasim Meshginfar, Alireza Sadeghi Mahoonak, Farah Hosseinian, Mohammad Ghorbani, Apollinaire Tsopmo
Journal of Food Science and Technology.2018; 55(9): 3498. CrossRef - Antidiabetic and antioxidant activities of brown and red macroalgae from the Persian Gulf
Kiana Pirian, Soheila Moein, Jelveh Sohrabipour, Reza Rabiei, Jaanika Blomster
Journal of Applied Phycology.2017; 29(6): 3151. CrossRef - UPLC-ESI-MS/MS and HPTLC Method for Quantitative Estimation of Cytotoxic Glycosides and Aglycone in Bioactivity Guided Fractions of Solanum nigrum L.
Karishma Chester, Sarvesh Paliwal, Washim Khan, Sayeed Ahmad
Frontiers in Pharmacology.2017;[Epub] CrossRef - Optimization of extraction of antioxidative phenolic compounds from cashew (Anacardium occidentale
L.) leaves using response surface methodology
Lalita Chotphruethipong, Soottawat Benjakul, Kongkarn Kijroongrojana
Journal of Food Biochemistry.2017; 41(4): e12379. CrossRef - Chemical Composition, Anti-bacterial and Cytotoxic Potential of n-Hexane Soluble Fraction of Monotheca buxifolia (Falc) A. DC. Fruit
Irfan Ullah, Jamshaid Ali Khan, Zafar Iqbal, Peer Abdul Hannan, Fazli Nasir, Salar Muhammad, Saqib Jahan, Mehreen Rehman
National Academy Science Letters.2017; 40(6): 405. CrossRef - Protective effects of Monotheca buxifolia fruit on renal toxicity induced by CCl4 in rats
Shumaila Jan, Muhammad Rashid Khan
BMC Complementary and Alternative Medicine.2016;[Epub] CrossRef - Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities
Waqas Khan Kayani, Erum Dilshad, Tanveer Ahmed, Hammad Ismail, Bushra Mirza
BMC Complementary and Alternative Medicine.2016;[Epub] CrossRef - Antioxidant and anti-inflammatory activites of Clerodendrum leaf extracts collected in Thailand
Narumol Phosrithong, Nantana Nuchtavorn
European Journal of Integrative Medicine.2016; 8(3): 281. CrossRef - Pharmacological screening of Monotheca buxifolia (Falc.) A. DC. for antinociceptive, anti-inflammatory and antipyretic activities
Irfan Ullah, Jamshaid Ali Khan, Muhammad Shahid, Ajmal Khan, Achyut Adhikari, Peer Abdul Hannan, Ibrahim Javed, Faisal Shakeel, Umar Farooq
BMC Complementary and Alternative Medicine.2016;[Epub] CrossRef - Studies on phytochemical, antioxidant, anti-inflammatory and analgesic activities of Euphorbia dracunculoides
Muhammad Majid, Muhammad Rashid Khan, Naseer Ali Shah, Ihsan Ul Haq, Muhammad Asad Farooq, Shafi Ullah, Anam Sharif, Zartash Zahra, Tahira Younis, Moniba Sajid
BMC Complementary and Alternative Medicine.2015;[Epub] CrossRef - Improvement of gel properties of sardine (Sardinella albella) surimi using coconut husk extracts
Natchaphol Buamard, Soottawat Benjakul
Food Hydrocolloids.2015; 51: 146. CrossRef - Comparative Analysis of Phenolics, Flavonoids, and Antioxidant and Antibacterial Potential of Methanolic, Hexanic and Aqueous Extracts from Adiantum caudatum Leaves
Dildar Ahmed, Muhammad Khan, Ramsha Saeed
Antioxidants.2015; 4(2): 394. CrossRef - Remedial Hypoglycemic Activity of n-Hexane Fraction of Hydro-Methanol Extract of Seed ofSwietenia mahagoni(L.) jacq. in Streptozotocin-Induced Diabetic Rat: A Comparative Evaluation
Tushar Kanti Bera, Kausik Chatterjee, Debidas Ghosh
Journal of Herbs, Spices & Medicinal Plants.2015; 21(1): 38. CrossRef - Nutritional and nutraceutical characteristics of Sageretia theezans fruit
Tae Kyung Hyun, Sang Churl Song, Chang-Khil Song, Ju-Sung Kim
Journal of Food and Drug Analysis.2015; 23(4): 742. CrossRef - Psidium cattleianum fruit extracts are efficient in vitro scavengers of physiologically relevant reactive oxygen and nitrogen species
Alessandra Braga Ribeiro, Renan Campos Chisté, Marisa Freitas, Alex Fiori da Silva, Jesuí Vergílio Visentainer, Eduarda Fernandes
Food Chemistry.2014; 165: 140. CrossRef


 PubReader
PubReader Cite
Cite
