Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 1(1); 2010 > Article
-
Original Article
Serum Homocysteine and Folate Levels are Associated With Late-life Dementia in a Korean Population - Ju Hee Song1, Moon Ho Park2, Changsu Han3, Sangmee A. Jo1, Kyungsook Ahn1
-
Osong Public Health and Research Perspectives 2010;1(1):17-22.
DOI: https://doi.org/10.1016/j.phrp.2010.12.006
Published online: December 7, 2010
1Division of Brain Diseases, Department of Biomedical Sciences, National Institute of Health, Korea Centers for Disease Control and Prevention, Seoul, Korea
2Departments of Neurology, Korea University Medical College, Ansan, Korea
3Psychiatry, Korea University Medical College, Ansan, Korea
- ∗Corresponding authors. Division of Brain Diseases, Department of Biomedical Sciences, National Institute of Health, Korea Centers for Disease Control & Prevention, Tongil-ro, Eunpyeong-gu, Seoul 122-701, Korea. asteroclear@gmail.com
© 2010 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives
- We aimed to determine whether serum levels of homocysteine (Hcy) and its biological determinants, folate and vitamin B12, are related to cognitive decline in elderly people.
-
Methods
- The concentrations of total Hcy, folate, and vitamin B12 were measured in serum samples from 424 cognitively normal controls, 382 mild cognitive impairment patients, and 56 dementia patients from Ansan Geriatric cohort. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery was used to evaluate cognitive functions.
-
Results
- The dementia patients had higher serum Hcy (dementia, 17.6 ± 6.9 μmol/L; control, 12.9 ± 5.0 μmol/L; p < 0.001) and lower serum folate (dementia, 7.9 ± 4.8 ng/mL; control, 10.0 ± 7.1 ng/mL; p = 0.034) levels compared with controls. There was an inverse relationship between Hcy levels and serum folate or vitamin B12 concentrations. The cognitive status as measured by the (CERAD) score was inversely related to Hcy levels. The adjusted odds ratio of dementia was 5.18 (95% confidence interval: 1.91–14.10; p = 0.001) for moderate (30 ≥ Hcy > 15) hyperhomocysteinemia compared with normal Hcy levels (≤15 μmol/L). In addition, there was weak association between low serum folate (<3.0 ng/mL) and the risk for dementia (crude odds ratio = 3.68; 95% confidence interval: 1.07–12.69; p = 0.039).
-
Conclusion
- Elevated serum Hcy and decreased serum folate concentrations are associated with the risk of dementia in Korean elders.
- Memory loss, dementia, and Alzheimer’s disease (AD) are major public health concerns worldwide, especially with the rapid increase in life expectancy in recent years. Late-life dementia is characterized by a progressive loss of memory and intellectual ability, also known as cognitive function. With the goal of finding effective strategies for prevention and treatment of cognitive impairment with aging, many researchers are now focusing on finding risk factors and biochemical markers that are associated with late-life dementia.
- Folate, vitamin B12, and homocysteine (Hcy) are involved in one-carbon transfer reactions essential for the maintenance of normal metabolic functions in the brain. Hcy is derived from the essential amino acid methionine through demethylation and can be remethylated to methionine. Its metabolism depends on the B vitamins cobalamin (vitamin B12), pyridoxine (vitamin B6), and folic acid (vitamin B9).1 Low levels of these vitamins, mutations of the 5,10-methylenetetrahydrofolate reductase gene, and renal function deficiency are associated with increased homocysteinemia.2 High Hcy suppresses cellular levels of S-adenosylmethionine and S-adenosylhomocysteine, interrupting the methylation of some functional proteins and genes. The mechanism of how hyperhomocysteinemia causes its neurotoxic effects is not fully understood.3
- Hcy has been identified as a peripheral marker directly relevant to oxidative stress that reflects neuropathy and vascular dysfunction.4,5 Epidemiology and clinical studies have demonstrated a positive correlation between elevated blood Hcy and the occurrences of dementia and AD; thus, hyperhomocysteinemia has been proposed to be a strong and independent risk factor for dementia.6–8 Other studies, however, have found no association between plasma Hcy levels and AD.9 Hyperhomocysteinemia has been identified in other neurological disorders, including Parkinson’s disease10 and brain atrophy.11
- Folate is required in the synthesis of S-adenosylmethionine, a methyl donor for many important brain biomolecules such as phospholipids, guanidinoacetate, neurotransmitters, amino acids, and nucleic acids.1 Among other B vitamins, folate has received much attention recently because its low serum level is found to be closely associated with structural and functional abnormalities in the brain. Low serum folate levels have been related to atrophy of the cerebral cortex,12 dementia,13 and cerebrovascular diseases.14
- Hcy accumulates in folate- and vitamin B12-deficient patients. Reductions in Hcy levels can be achieved with high doses of folic acid, vitamin B12, and vitamin B6 in the general population15 and in individuals with AD.16 Supplementation with folate has resulted in a positive effect on cognitive functions and memory deficits.17,18 Therefore, hyperhomocysteinemia and deficiencies in folate and vitamin B12 may contribute to the pathogenesis of cognitive impairment. However, a recent clinical trial reported that the regimen of high-dose B vitamin supplements was effective in reducing Hcy but did not slow cognitive decline in individuals with mild to moderate AD.19 Thus, it is important to reveal whether there are relationships between cognition function, levels of Hcy, folate, and vitamin B12, and dementia, because they could plausibly represent a disease-modifying intervention in dementia.
- In the present study, we analysed serum samples of 862 participants from Ansan Geriatric cohort to determine whether serum levels of Hcy, folate, and vitamin B12 are related to cognitive functioning and the risk for dementia.
Introduction
- Participants aged 60 years or older were enrolled in the Ansan Geriatric cohort study at the Geriatric Health Clinic and Research Institute, Korea University Medical Center, Ansan City, South Korea. A total of 862 participants were recruited from April 2006 to January 2008 and were evaluated by clinical and neuropsychological examinations after full medical history and physical assessments.20
- Written informed consent was obtained from all subjects after the nature of the study and its procedures had been explained. The research protocols were reviewed and approved by the institutional review boards of the Korea Centers for Disease Control and Prevention and the Medical College of Korea University.
- Cognitive functioning and memory impairments of subjects were assessed using a Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery,21 which included the following tests: Word Fluency Test for animal categories, the 15-item Korean version of the Boston Naming Test, the Korean version of the mini-mental state examination (MMSE), Word List Memory test, Constructional Praxis test, Word Delayed Recall, Word Recognition, and Construction Recall. The total CERAD score was used as an index for the overall cognition level of the participants.22 All participants were clinically evaluated according to published guidelines, and each dementia patient met the criteria for the Diagnostic and Statistical Manual of Mental Disorders, fourth edition.23 Mild cognitive impairment (MCI) was diagnosed based on the Mayo Clinic criteria.24
- Blood samples were collected in serum separator tube II and incubated for 30–45 minutes at room temperature to allow clotting. The serum was then separated by centrifugation, kept in a refrigerator (−4°C), and analysed within 1 day. Serum Hcy concentrations were measured by a competitive immunoassay using a direct chemiluminescence method (ADVIA Centaur® immunoassay system, Bayer Healthcare, Tarrytown, NY, USA).25 Serum folate and vitamin B12 concentrations were determined by competitive binding assays using a Simul TRAC-SNB radioimmunoassay kit (ICN Biomedicals, Ausora, Ohio, USA).
- Statistical analysis of the mean values of different variables from AD, MCI, and control groups was performed by analysis of covariance using a general linear model. A χ2 test was used to compare the frequencies of ApoE4 carriers and male:female ratios among AD, MCI, and control groups. Associations between CERAD scores and Hcy, folate, and vitamin B12 serum levels were evaluated using Pearson’s correlation analysis. Because age, gender, and duration of education were significantly different in dementia and controls, these variables were adjusted. Multivariable-adjusted logistic regression analysis was conducted to examine the odds ratio (OR) for dementia across the range of Hcy and folate levels. Covariate variables were age, gender, and duration of education, which were potential confounding factors for cognitive function. Statistical significance was considered at p < 0.05. SAS software release 9.1 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis.
Material and Methods
- Demographic characteristics of the study subjects are shown in Table 1. Of the 862 elderly Koreans examined, 382 (44.3%) had MCI and 56 (6.5%) were dementia patients. The dementia patients were older and less educated than the controls. There was a significant difference in the male:female ratio among the three diagnostic groups. The average age of the total participants was 71.1 ± 5.2 years, and 56.0% of them were female. Serum folate, vitamin B12, and Hcy levels were measured in all 862 subjects. Apolipoprotein E (ApoE) genotype data were available for 807 (93.6%) participants, and the CERAD score was available for 805 (93.4%).
- We found significantly increased Hcy (17.6 ± 6.9 μmol/L) serum concentrations in dementia patients compared with healthy controls (13.0 ± 5.1 μmol/L, p < 0.001) or MCI patients (13.3 ± 4.8 μmol/L, p < 0.001) and no significant difference between MCI and controls (p = 0.375). The differences remained significant after adjusting for age, gender, and duration of education.
- The serum folate and vitamin B12 levels were significantly lower in dementia patients compared with controls with adjusted p values of 0.05 for folate and 0.01 for vitamin B12. The folate and vitamin B12 levels of the MCI group were lower than those of the controls and were without statistical significance. However, vitamin B12 levels of dementia patients were significantly lower than those of the MCI group (p < 0.001).
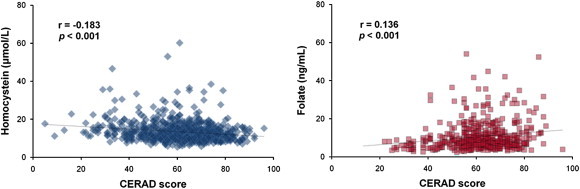
- Mean MMSE and CERAD scores were highest in controls and lowest in dementia patients. Serum levels of Hcy were inversely correlated with the CERAD score (r = −0.183; p < 0.001), and folate levels were positively correlated with CERAD score (r = 0.136; p < 0.001) (Figure 1). Multiple regression analysis after adjustment for age and gender showed that serum levels of vitamin B12 and folate are associated with CERAD as well as MMSE scores (p < 0.01). There was an inverse correlation between serum Hcy and folate levels (r = −0.227, p < 0.001, adjusted for age, gender, and education).
- None of the subjects in this study had severe hyperhomocysteinemia (Hcy > 100 μmol/L); therefore, the subjects were classified into three groups according to serum Hcy concentrations: intermediate (>30 μmol/L), moderate (30 ≥ Hyt > 15), and normal (≤15) hyperhomocysteinemias.26 The folate levels were categorized as low (<3.0 ng/mL), normal (3.0 ≤ folate < 17.0), and high (≥17.0) groups27 as shown in Table 2.
- A total of 192 subjects (22.3%) had moderate and 17 (1.9%) had intermediate hyperhomocysteinemia. The group of subjects with hyperhomocysteinemia consisted of a higher percentage of males, were older, had lower levels of folate and vitamin B12, and had a higher prevalence of dementia (p < 0.001) but not of MCI. Only about 3% (26 subjects) had low folate levels but had higher prevalence of dementia and MCI. Because depression is frequently accompanied by cognitive impairment in elders, we accessed the depressive symptoms of the study participants using a Korean version of the 30-item Geriatric Depression Scale. The presence of depressive symptoms did not affect serum Hcy, folate, or vitamin B12 concentrations in our study subjects.
- To assess the risk of dementia according to serum Hcy and folate concentrations, multivariable logistic regression analysis was performed. Because age, gender, duration of education, and levels of Hcy, folate, vitamin B12 were significantly different in dementia and controls, these variables were included as covariates (Table 3). Moderate hyperhomocysteinemia (30 μmol/L ≥ Hyt > 15 μmol/L) showed an increased risk for dementia with a crude OR of 4.56. After adjusting for covariates, the OR was increased to 5.18 (p = 0.001). The intermediate hyperhomocysteinemia (>30 μmol/L) diagnosed in 1.9% (17 subjects) of the participants had much higher risk for dementia (adjusted OR = 15.59, p = 0.007). Furthermore, low levels of serum folate (<3.0 ng/mL) had an increased risk for dementia (crude OR = 3.68, p = 0.039), but adjusted OR was not significant. High folate levels (≥17.0 ng/mL) did not show a protective effect. With respect to associations with MCI, the high folate levels showed protective effect against MCI (crude OR = 0.63, p = 0.045), but the effect was not significant after adjusting for the confounding factors (p = 0.312). The Hcy levels were not associated with the risk for MCI.
Results
- The main outcome of this cross-sectional study was the significant differences in serum Hcy, folate, and vitamin B12 concentrations between dementia patients and healthy controls. In addition, elevated Hcy and decreased folate levels were correlated with a decrease in cognitive functioning as measured by CERAD and MMSE scores. Hcy concentrations higher than 15 μmol/L increased the risk of dementia by 5.7 times the Hcy level of 15 μmol/L or lower (adjusted OR = 5.69; 95% confidence interval: 2.13–15.22; p < 0.001).
- In accordance with previous studies, our results showed that serum Hcy levels increased with age and were higher in males than in females and that Hcy and folate levels are associated with cognitive status or risk for dementia.6,8 A similar study was reported from a prospective Kwangju community survey, which showed that incident dementia is strongly associated with lower folate and vitamin B12 concentrations and higher Hcy concentrations in Korean elderly people.28 However, some studies observed no relationship between Hcy levels and cognitive impairment.9 Among community-dwelling elderly subjects aged 55 years and older from the population-based Rotterdam study in the Netherlands, no relationship was found between Hcy levels and concurrent cognitive impairments, as assessed by MMSE, or subsequent cognitive decline over 2.7 years.29
- The association between elevated Hcy levels and dementia may be explained by several mechanisms, including neurotoxic effect of Hcy. A number of findings demonstrate that the central nervous system is acutely sensitive to Hcy, probably because of its stimulating effects on calcium influx and promotion of glutamate excitotoxicity as well as its role in reactive oxygen species-mediated insults.2 A recent animal study showed that hyperhomocysteinemia could increase beta amyloid production through the enhanced expression of γ-secretase complex and amyloid precursor protein phosphorylation, causing memory deficits that could be rescued by folate and vitamin B12 treatment30 suggesting a plausible link between Hcy and the pathogenesis of AD. Although the generation and toxicity of Hcy are not completely understood, it might be beneficial to use Hcy as a biomarker for cognitive decline.
- High Hcy has been correlated with decreased brain volume. A recent clinical trial showed that the supplementation of high-dose B vitamins to MCI patients for 2 years reduced Hcy levels and had beneficial effects on brain shrinkage.31 An interesting observation was that B vitamin treatment slowed the rate of brain atrophy and memory decline only in the group with initial Hcy level higher than 13 μmol/L (top quartile) but not in the group with the initial Hcy level below 9.5 μmol/L (lowest quartile). The results suggest that the treatment with Hcy-lowering B vitamins could be an effective way of delaying onset of dementia for those with moderate hyperhomocysteinemia.
- Numerous studies have shown that the elevated blood Hcy is associated with impaired cognition and a higher risk of dementia.5–8 In this study, we focused on comparing the serum Hcy, folate, and vitamin B12 levels in association with the risk for MCI or dementia. One limitation of this study is that we did not include the analyses of other confounding factors that might influence the incidence of dementia, such as alcohol consumption, smoking, and exercise habits. Although we observed weak association between drinking, smoking, and exercise habits and prevalence of MCI or dementia, some of the data were not reliable in that they were obtained from self-reports of the patients and some of the data were not available. Another limitation of this study is that this is a cross-sectional study that could not address causal relation between Hcy level and cognitive impairment. Despite these limitations, the association between Hcy and cognitive decline is consistent with previously published data. Further follow-up studies are needed to examine whether serum Hcy level could be used as a peripheral biomarker for cognitive decline and onset of dementia.
- In conclusion, the apparently strong association of high Hcy levels with the risk of dementia and cognitive impairment suggests that hyperhomocysteinemia and low folate levels may serve as independent risk factors for dementia in Koreans. Further efforts are needed to identify at-risk elderly population and targets for early intervention to reduce the risk of late-life dementia.
Discussion
-
Acknowledgements
- This study was supported by grants from the National Institute of Health, Korea (091-4845-300-210; 091-4845-300-260).
Acknowledgements
-
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article information
- 1. Stipanuk M.H.. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24:2004;539−577. PMID: 15189131.ArticlePubMed
- 2. Marlatt M.W., Lucassen P.J., Perry G.. Alzheimer’s disease: cerebrovascular dysfunction, oxidative stress, and advanced clinical therapies. J Alzheimers Dis 15:2008;199−210. PMID: 18953109.ArticlePubMedPMC
- 3. Maron B.A., Loscalzo J.. The treatment of hyperhomocysteinemia. Annu Rev Med 60:2009;39−54. PMID: 18729731.ArticlePubMedPMC
- 4. Hankey G.J.. Is plasma homocysteine a modifiable risk factor for stroke? Nat Clin Pract Neurol 2:2006;26−33. PMID: 16932518.ArticlePubMed
- 5. Boushey C.J., Beresford S.A., Omenn G.S., Motulsky A.G.. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 274:1995;1049−1057. PMID: 7563456.ArticlePubMed
- 6. Clarke R., Smith A.D., Jobst K.A.. Folate, vitamin B12, and serum total levels in confirmed Alzheimer’s disease. Arch Neurol 55:1998;1449−1455. PMID: 9823829.ArticlePubMed
- 7. Obeid R., Herrmann W.. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580:2006;2994−3005. PMID: 16697371.ArticlePubMed
- 8. Seshadri S., Beiser A., Selhub J.. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346:2002;476−483. PMID: 11844848.ArticlePubMed
- 9. Mizrahi E.H., Jacobsen D.W., Debanne S.M.. Plasma total homocysteine levels, dietary vitamin B6, and folate intake in AD and healthy aging. J Nutr Health Aging 7:2003;160−165. PMID: 12766793.PubMed
- 10. Muller T., Werne B., Fowler B., Kuhn W.. Nigral endothelial dysfunction, homocysteine, and Parkinson’s disease. Lancet 354:1999;126−127. PMID: 10408491.ArticlePubMed
- 11. Sachdev P.S., Valenzuela M., Wang X.L.. Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology 58:2002;1539−1541. PMID: 12034795.ArticlePubMed
- 12. Snowdon D.A., Tully C.L., Smith C.D.. Serum folate and the severity of atrophy of the neocortex in Alzheimer’s disease: findings from the Nun study. Am J Clin Nutr 71:2000;993−998. PMID: 10731508.ArticlePubMed
- 13. Ramos M.I., Allen L.H., Mungas D.M.. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 82:2005;1346−1352. PMID: 16332669.ArticlePubMed
- 14. Maxwell C.J., Hogan D.B., Ebly E.M.. Serum folate levels and subsequent adverse cerebrovascular outcomes in elderly persons. Dement Geriatr Cogn Disord 13:2002;225−234. PMID: 12006733.ArticlePubMed
- 15. Clark R., Frost C., Leroy V., Collins R.. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 316:1998;894−898. PMID: 9569395.ArticlePubMedPMC
- 16. Aisen P.S., Egelko S., Andrews H.. A pilot study of vitamins to lower plasma homocysteine levels in Alzheimer’s disease. Am J Geriatr Psychiatry 11:2003;246−249. PMID: 12611755.ArticlePubMed
- 17. Durga J., van Boxtel M.P., Schouten E.G.. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 369:2007;208−216. PMID: 17240287.ArticlePubMed
- 18. Nilsson K., Gustafson L., Hultberg B.. Improvement of cognitive functions after cobalamin/folate supplementation in elderly patients with dementia and elevated plasma homocysteine. Int J Geriatr Psychiatry 16:2001;609−614. PMID: 11424170.ArticlePubMed
- 19. Aisen P.S., Schneider L.S., Sano M.. High-dose B vitamin supplementation and cognitive decline in Alzheimer’s disease: a randomized controlled trial. JAMA 300:2008;1774−1783. PMID: 18854539.ArticlePubMedPMC
- 20. Han C., Jo S.A., Kim N.H.. Study design and methods of the Ansan Geriatric Study (AGE study). BMC Neurol 9:2009;10PMID: 19236723.ArticlePubMedPMC
- 21. Lee J.H., Lee K.U., Lee D.Y.. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci 57:2002;47−53.Article
- 22. Chandler M.J., Lacritz L.H., Hynan L.S.. A total score for the CERAD neuropsychological battery. Neurology 65:2005;102−106. PMID: 16009893.ArticlePubMed
- 23. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition. 1994. American Psychiatric Press; Washington.
- 24. Petersen R.C., Smith G.E., Waring S.C.. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:1999;303−308. PMID: 10190820.ArticlePubMed
- 25. Mindicino H.J., Carlsen J., Tewari P.. An evaluation of an automated homocysteine method on the Bayer ADVIA Centaur automated chemiluminescent system. Clin Lab 48:2002;493−496. PMID: 12389709.PubMed
- 26. Ravaglia G., Forti P., Maioli F.. Homocysteine and cognitive function in healthy elderly community dwellers in Italy. Am J Clin Nutr 77:2003;668−673. PMID: 12600859.ArticlePubMed
- 27. Bottigleri T.. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev 54:1996;382−390. PMID: 9155210.ArticlePubMed
- 28. Kim J.M., Stewart R., Kim S.W.. Changes in folate, vitamin B12, and homocysteine associated with incident dementia. J Neurol Neurosurg Psychiatry 79:2008;864−868. PMID: 18252751.ArticlePubMed
- 29. Kalmijn S., Launer L.J., Lindemans J.. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol 150:1999;283−289. PMID: 10430233.ArticlePubMed
- 30. Zhang C.E., Wei W., Liu Y.H.. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol 174:2009;1481−1491. PMID: 19264913.ArticlePubMedPMC
- 31. Smith A.D., Smith S.M., de Jager C.A.. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One 5:2010;e12244PMID: 20838622.ArticlePubMedPMC
References
Values are mean ± SD. The p values were calculated using analysis of covariance or χ2 test (male/female, ApoE4 allele). Bold values are p < 0.05.
MCI = mild cognitive impairment; MMSE = mini-mental state examination; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; SD = standard deviation.
The p values were calculated using analysis of covariance or χ2 test. Bold values are p < 0.05.
The concentration ranges for Hcy and folate were set according to Refs 26 and 27, respectively.
Hcy = homocysteine; Fol = folate; MCI = mild cognitive impairment; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease.
Model 1: adjusted for age, gender, and years of education. Model 2: adjusted for age, gender, years of education, vitamin B12, and folate (or homocysteine).
The reference concentrations were Hcy ≤ 15 μmol/L and 3.0 ng/mL ≤ Folate < 17.0 ng/mL. Bold values are p < 0.05.
MCI = mild cognitive impairment; Hcy = homocysteine.
Figure & Data
References
Citations

- B vitamins and prevention of cognitive decline and incident dementia: a systematic review and meta-analysis
Zhibin Wang, Wei Zhu, Yi Xing, Jianping Jia, Yi Tang
Nutrition Reviews.2022; 80(4): 931. CrossRef - Biochemical and Hematological Correlates of Elevated Homocysteine in National Surveys and a Longitudinal Study of Urban Adults
May A. Beydoun, Hind A. Beydoun, Peter H. MacIver, Sharmin Hossain, Jose A. Canas, Michele K. Evans, Alan B. Zonderman
Nutrients.2020; 12(4): 950. CrossRef - Hyperhomocysteinemia is key for increased susceptibility to PND in aged mice
Guangchao Zhao, Jiao Deng, Yuan Shen, Peng Zhang, Hailong Dong, Zhongcong Xie, Lize Xiong
Annals of Clinical and Translational Neurology.2019; 6(8): 1435. CrossRef - The effects and potential mechanisms of folic acid on cognitive function: a comprehensive review
Athena Enderami, Mehran Zarghami, Hadi Darvishi-Khezri
Neurological Sciences.2018; 39(10): 1667. CrossRef


 PubReader
PubReader Cite
Cite
