Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 15(2); 2024 > Article
-
Brief Report
Gender differences in hepatitis A seropositivity rates according to the Republic of Korea’s vaccination policy -
Hyunjin Son1
 , Sunhyun Ahn2
, Sunhyun Ahn2 , Wonseo Park3
, Wonseo Park3 , Gayoung Chun3
, Gayoung Chun3 , Unyeong Go3
, Unyeong Go3 , Sang Gon Lee2
, Sang Gon Lee2 , Eun Hee Lee4
, Eun Hee Lee4
-
Osong Public Health and Research Perspectives 2024;15(2):168-173.
DOI: https://doi.org/10.24171/j.phrp.2023.0263
Published online: April 16, 2024
1Department of Preventive Medicine, Dong-A University College of Medicine, Busan, Republic of Korea
2Department of Laboratory Medicine, Green Cross Laboratories, Yongin, Republic of Korea
3Infectious Disease Research Center, Green Cross Laboratories, Yongin, Republic of Korea
4Green Cross Laboratories, Yongin, Republic of Korea
- Corresponding author: Sang Gon Lee Department of Laboratory Medicine, Green Cross Laboratories, 107 Ihyeon-ro 30beon-gil, Giheung-gu, Yongin 16924, Republic of Korea E-mail: sglee@gclabs.co.kr
- Co-Corresponding author: Eun Hee Lee Green Cross Laboratories, 107 Ihyeon-ro 30beon-gil, Giheung-gu, Yongin 16924, Republic of Korea E-mail: ehlee@gclabs.co.kr
© 2024 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 430 Views
- 18 Download
Abstract
-
Objectives
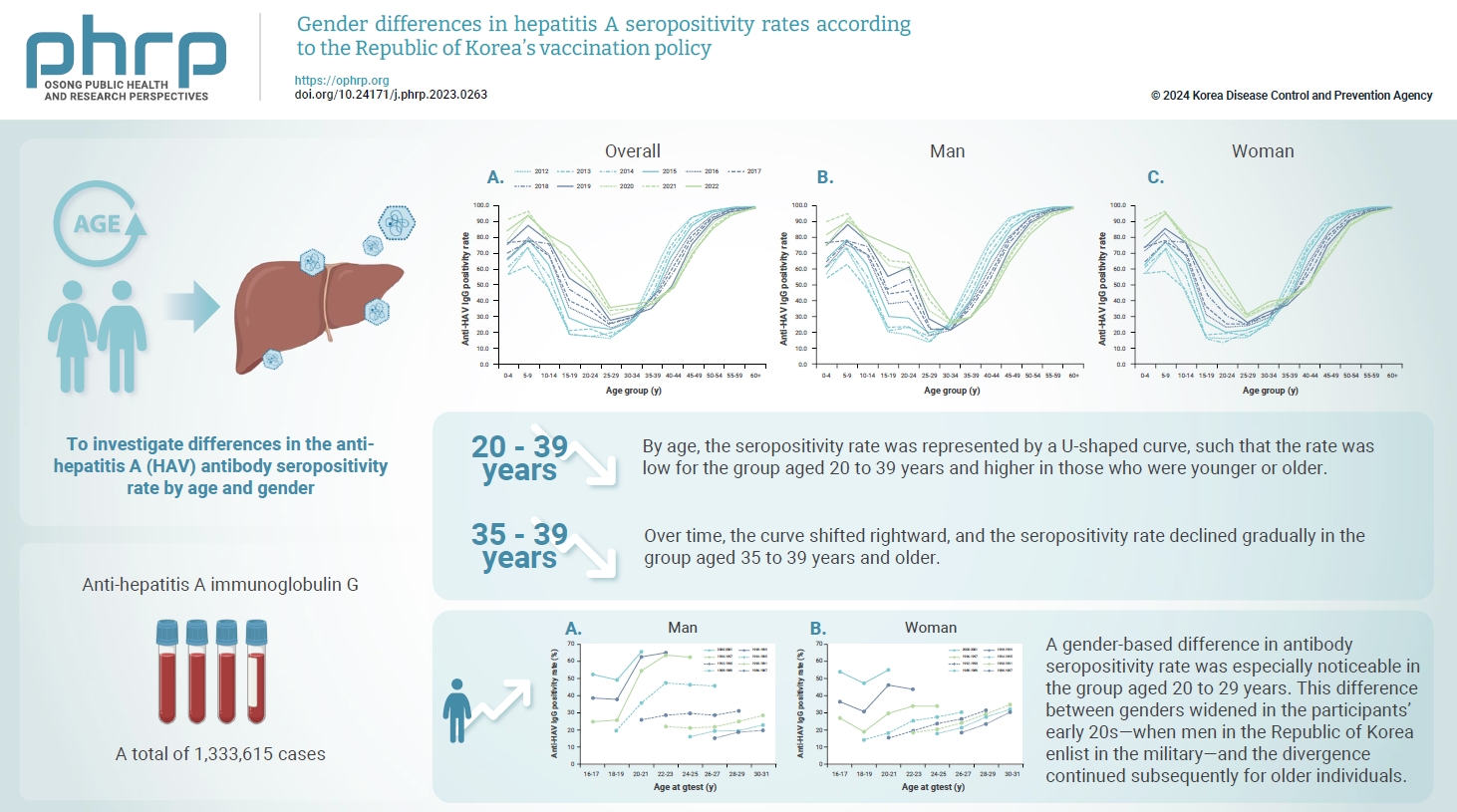
- This study aimed to investigate differences in the anti-hepatitis A virus (HAV) antibody seropositivity rate by age and gender.
-
Methods
- We collected information on anti-HAV immunoglobulin G and immunoglobulin M status from samples submitted for HAV antibody testing in 2012–2022. A total of 1,333,615 cases were included in the analysis.
-
Results
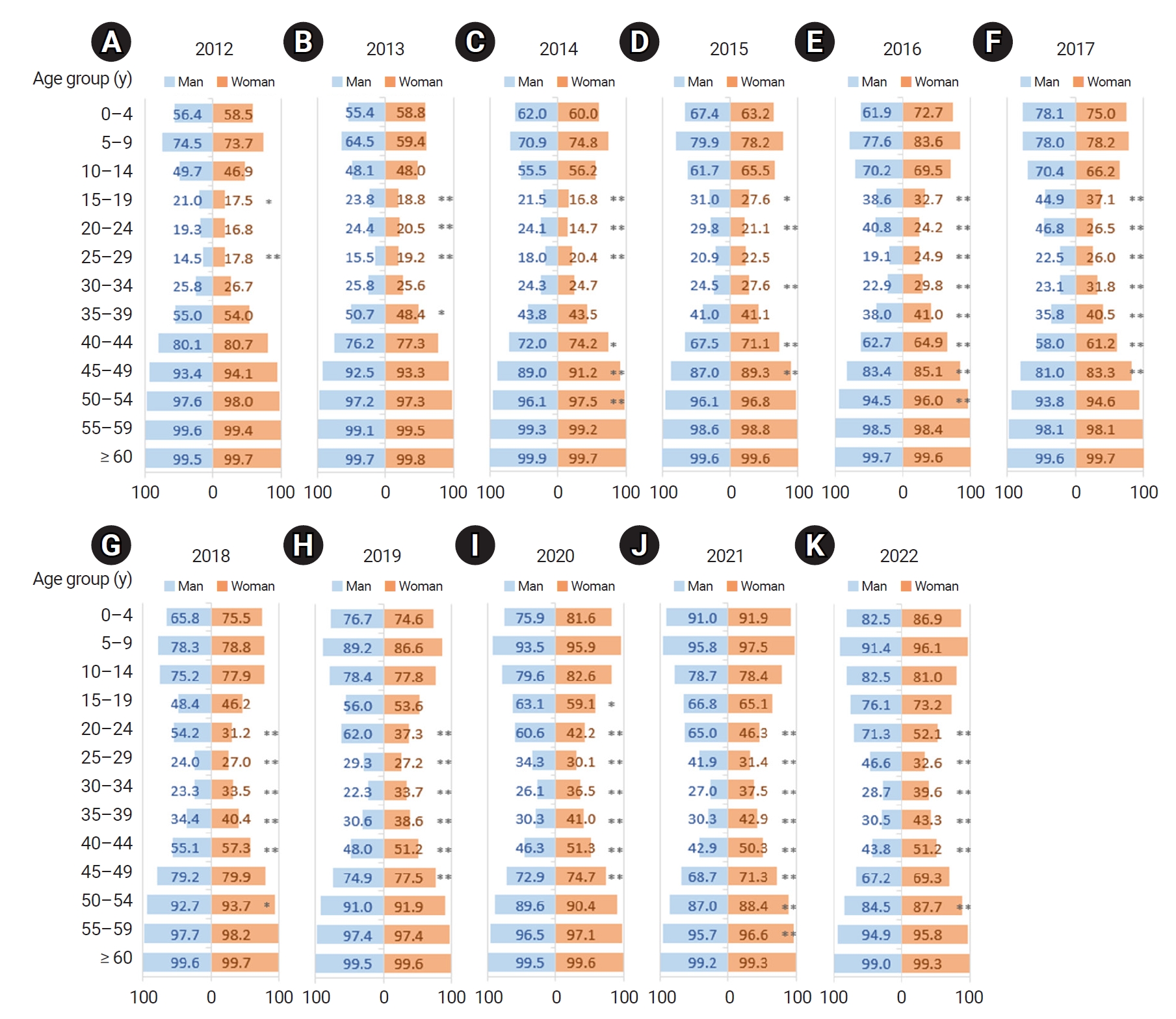
- By age, the seropositivity rate was represented by a U-shaped curve, such that the rate was low for the group aged 20 to 39 years and higher in those who were younger or older. Over time, the curve shifted rightward, and the seropositivity rate declined gradually in the group aged 35 to 39 years and older. A gender-based difference in antibody seropositivity rate was especially noticeable in the group aged 20 to 29 years. This difference between genders widened in the participants’ early 20s—when men in the Republic of Korea enlist in the military—and the divergence continued subsequently for older individuals.
-
Conclusion
- These results indicate a higher risk of severe infection among older individuals and a gender-based difference in seroprevalence. Therefore, it is necessary to implement policies to promote vaccination in adults.
- Hepatitis A virus (HAV) infections can be transmitted by the fecal-oral route, through close contact, or through contaminated food or water. In the Republic of Korea, a total of 17,598 cases of HAV (33.9 cases per 100,000 individuals) were reported in 2019 [1]. This is the largest epidemic since HAV was designated a notifiable infectious disease in 2010; contaminated salted clams were the primary source of infection [2–4].
- In 2019, 86.6% of the HAV patients reported in the Republic of Korea were aged 20 to 49 years [1], which may be attributable to differences in immunity, not exposure. According to the 2015 Korea National Health and Nutrition Examination Survey, the anti-HAV immunoglobulin G (IgG) seropositivity rate was lowest (11.9%) in the group aged 20 to 29 years and very high (97.8%) in the group aged ≥45 years [5].
- To understand the epidemiology of HAV infection, surveillance of anti-HAV IgG seroprevalence is important [6]. Anti-HAV IgG seroprevalence in the Republic of Korea has been studied extensively in the past [5,7,8]. Most studies focused primarily on the time-series changes in age-specific seroprevalence. Although the incidence of hepatitis A was higher among men traditionally, the gender ratio in hepatitis A incidence has changed in recent years. In particular, in 2019 the incidence ratio was inverted among individuals aged 20 to 29 years, showing a higher incidence among women than men [9].
- This gender-based difference in HAV incidence also seems to be attributable to differences in immunity, not exposure. All men in the Republic of Korea enlist in the military in their early 20s, and mandatory single-dose vaccination against hepatitis A has been enforced for this age group since 2012 [9–11]. The rapid spike in antibody seroprevalence among men aged 20 to 29 years may be considered a result of this policy.
- Therefore, this study aimed to investigate differences in the anti-HAV antibody seroprevalence rate by age and gender.
Introduction
- Study Population
- Samples submitted to the Green Cross Labs for HAV antibody testing between January 1, 2012, and June 30, 2022 were used in this study. Data regarding anti-HAV IgG and immunoglobulin M (IgM) status were collected. Cases lacking information about gender or age were excluded from the analysis; cases were also excluded when a positive result was confirmed from an anti-HAV IgM test performed simultaneously with an anti-HAV IgG test. A total of 1,333,615 cases were included in the analysis. Table 1 shows the distributions by year, gender, and age.
- Anti-HAV Testing
- HAV antibody (anti-HAV IgG, IgM) testing was performed with a Chemiluminescence microparticle immunoassay, using the Architect i2000 analyzer (Abbott, Singapore). The testing methods did not change significantly during the study period.
- Statistical Analysis
- The anti-HAV IgG positivity rate was determined by year, gender, and age (5-year units), and 95% confidence intervals (CIs) were determined using binomial exact distribution. To examine the difference in the anti-HAV IgG positivity rate by birth year in men and women, we presented the difference in seroprevalence over time in 2-year birth cohorts. Chi-square tests were used to compare seroprevalence between genders, with a significance level of p<0.05. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute).
- Ethics Statement
- The study was approved by the Institutional Review Board of Green Cross Laboratory (no: GCL-2022-1048-01).
Materials and Methods
- A total of 1,333,615 cases were analyzed in this study. The number of cases per year rose from 61,462 cases in 2012 to 179,009 cases in 2021. From January to June 2022, 79,131 cases were included in the study. Approximately 40.2% of the total cases were male, and 66.5% were aged 25 to 49 years (Table 1). Of the total, 86% were from requests for anti-HAV IgG testing alone, and 14% were from requests for both anti-HAV IgG and IgM testing. Table S1 present the anti-HAV IgG positivity rates and 95% CIs by year, gender, and 5-year units of age from January 2012 to June 2022.
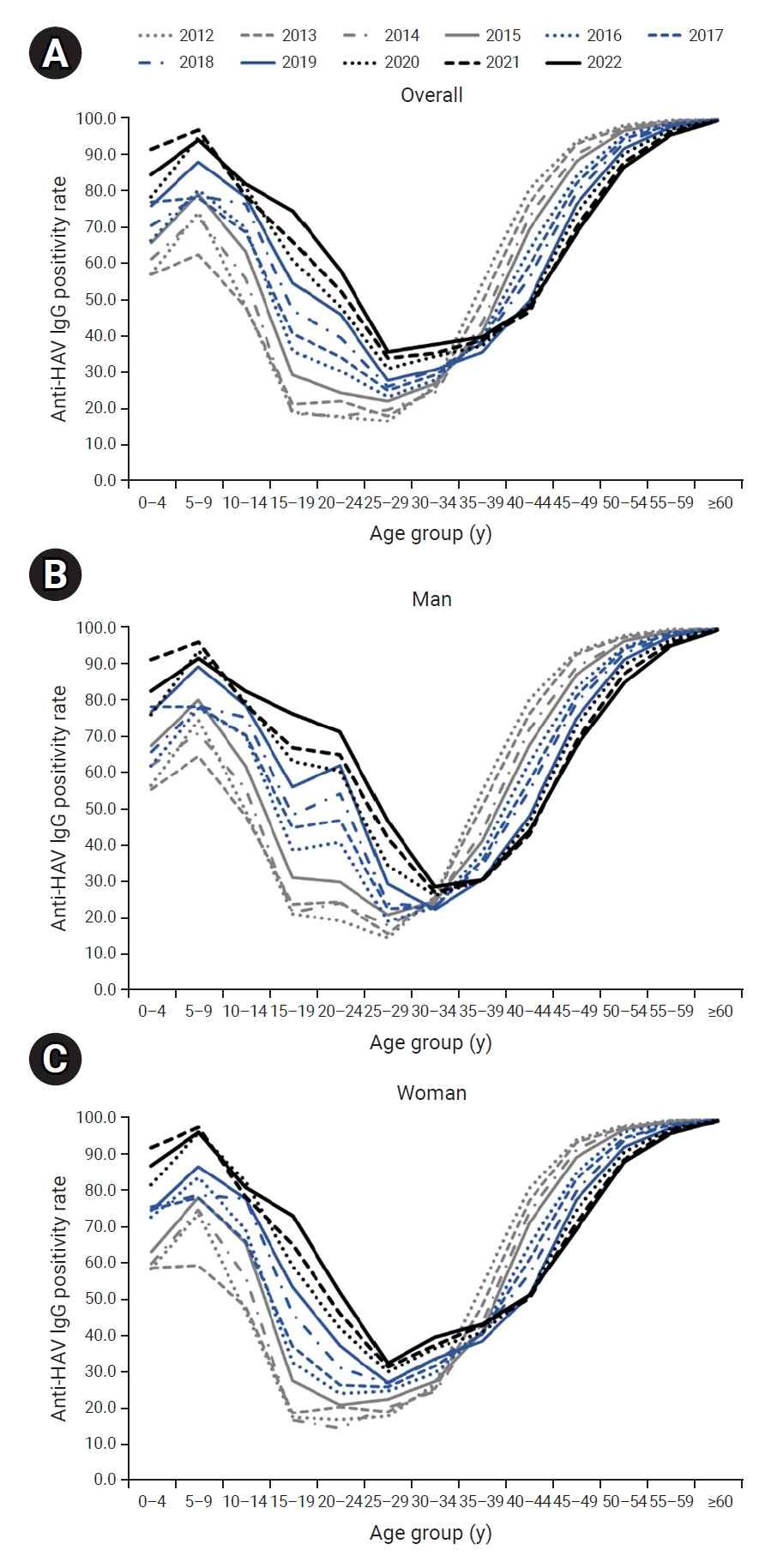
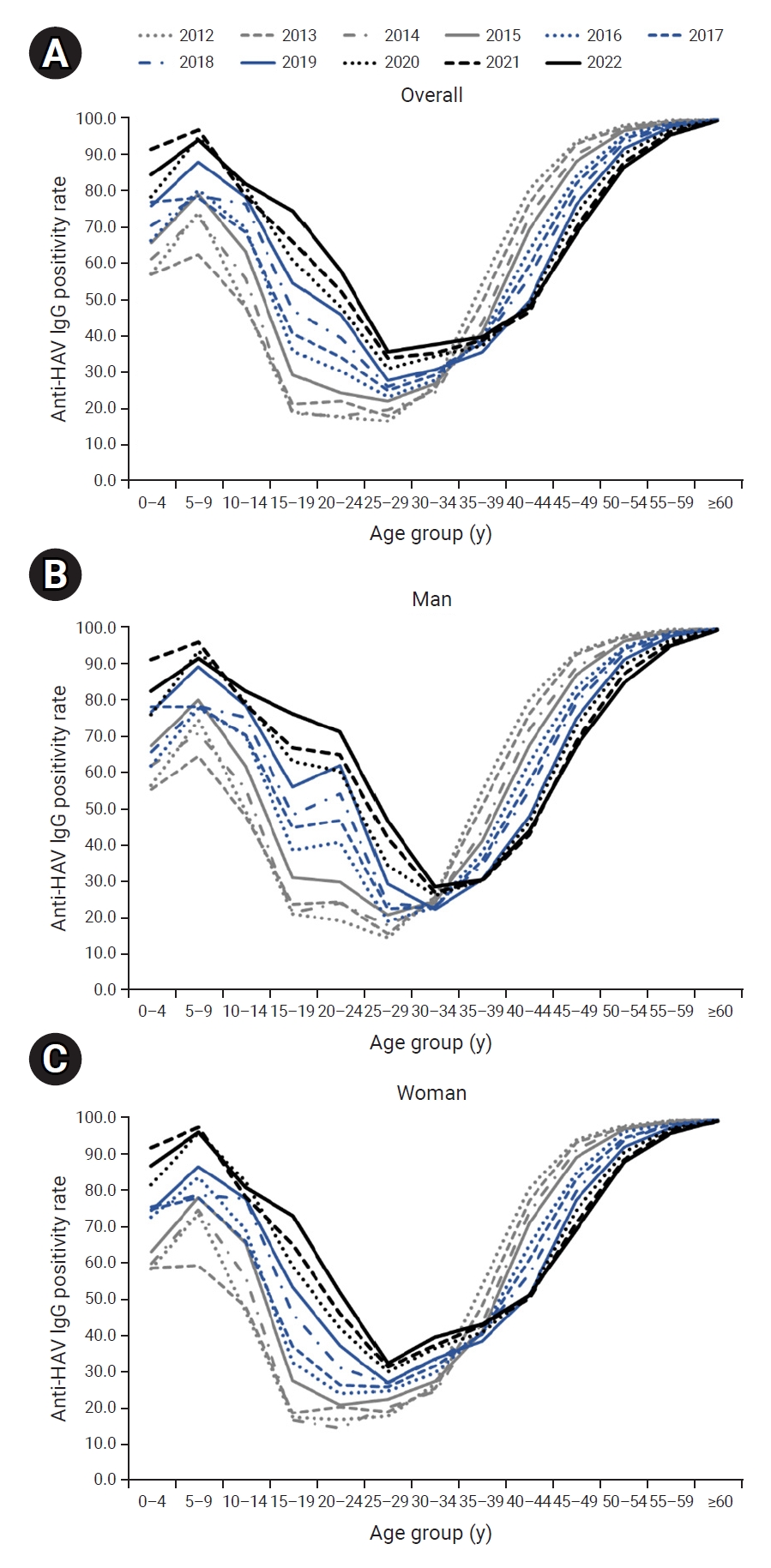
- The seropositivity rate by age formed a U-shaped curve, such that seroprevalence was low in the group aged 20 to 39 years and higher in the age intervals younger and older than this group. The seropositivity rate in the group aged 30 to 34 years and younger increased over time, while the rate in the group aged 35 to 39 years and older declined over time, resulting in a rightward shift during the study period (Figure 1A).
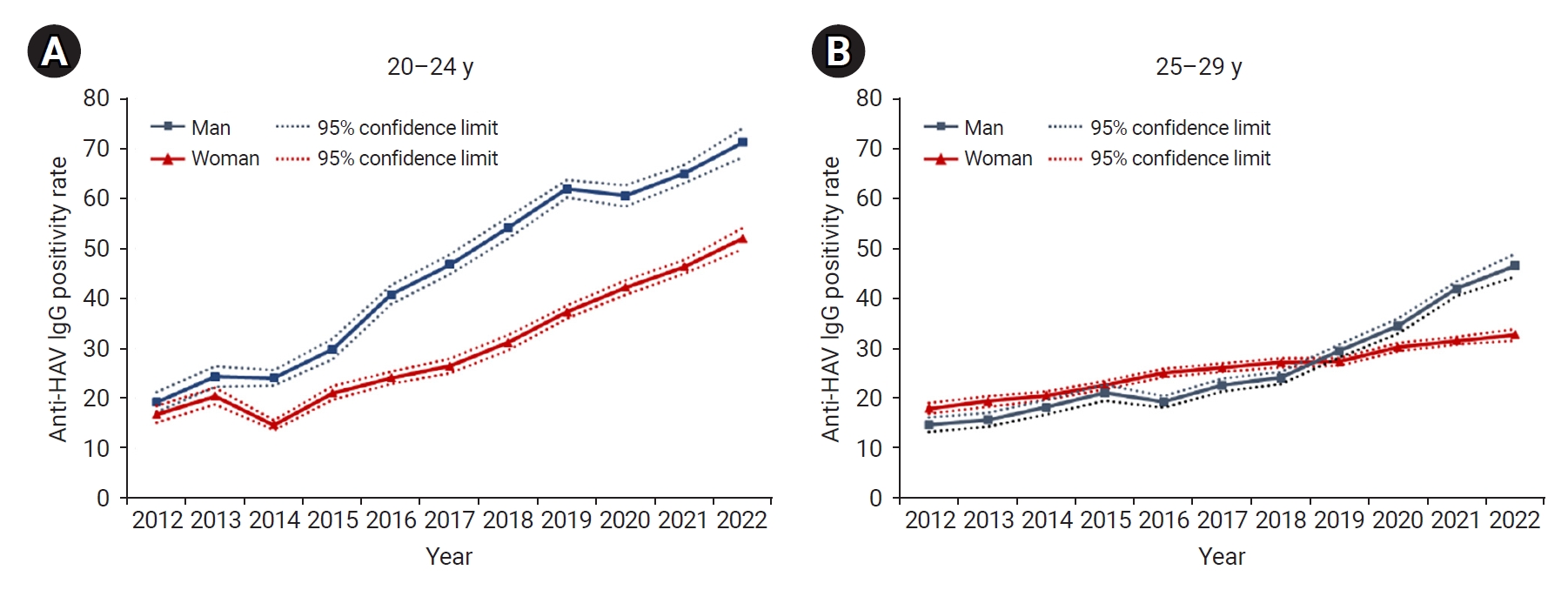
- The rightward shift of the U-shaped curve of the age- and year-specific seropositivity rate was evident even when the cases were separated by gender. However, the seropositivity rate in men aged 20 to 29 years was much higher than that in same-aged women (Figure 1B, C). As a result, in the most recent data in 2022, the seropositivity rate in men aged 30 years and older was relatively low, similar to the rate among women, whereas the seropositivity rate in men aged 20 to 29 years was significantly higher than the rate in women (Figure 2).
- Regarding the gender-based difference in seropositivity rate, the rate has diverged more widely by gender since 2014 in the group aged 20 to 24 years. Until 2018, in the group aged 25 to 29 years, the seropositivity rate was slightly higher among women, but after 2019, the rate was higher among men (Figure 3).
- Figure 4 illustrates the changes in seropositivity rate by 2-year birth cohorts. In both genders, the rate has risen in all ages across more recent birth cohorts, and within age groups, the rate increased in more recent cohorts. In particular, in the 1994–1995 and subsequent birth cohorts, a dramatic rise in the seropositivity rate occurred among men in the group aged 20 to 21 years.
Results
- Overall, the anti-HAV IgG positivity rate has risen from 2012 to 2022. The depth and width of the U-curve representing the age-specific seropositivity rate have decreased during that period. However, as the U-curve shifts rightward and the seropositivity rate declines gradually among individuals aged ≥40 years, the overall risk of severe infection may increase because of the nature of HAV infection, where the likelihood of severity increases with advancing age [12]. In the Republic of Korea, a hepatitis A vaccine was approved in 1997 and has been included in the national immunization program for infants and toddlers since 2015 [5]. For adults, vaccines are administered to individuals at high risk, but the target population is very small. These results indicate that additional policies must be implemented to facilitate the vaccination of adults [13].
- Antibody seroprevalence diverges dramatically by gender in the group aged 20 to 29 years. The divergence is particularly noticeable in participants in their early 20s, and it continues subsequently for older individuals. In the Republic of Korea, mandatory military service is required of adult men; therefore, all men enlist in the military in their early 20s. Additionally, hepatitis A vaccines have been provided to military recruits since 2012 [9–11]. Therefore, the inversion of the incidence rate between men and women, where the incidence of HAV infection among women has outpaced that of men since the year 2019 [9], may be a result of this vaccination policy. The gender-based difference in HAV antibody positivity rates and incidence rates is expected to continue. Furthermore, at 76.1%, the current (2022) HAV antibody seropositivity rate is very high among men aged 15 to 19 years. This is because the vaccination rate has gradually increased in the cohort born after the hepatitis A vaccine was approved in the Republic of Korea in 1997 and began to be used in the private market [5]. Therefore, the current policy for mandatory hepatitis A vaccination for military enlistees must be reviewed and amended.
- This study had some limitations. The data used in this study contain information only on gender and age, so we could not examine differences in seropositivity rate according to socioeconomic status. Furthermore, probability sampling was not performed. Nevertheless, one of the strengths of this study is that it examined changes in anti-HAV IgG seropositivity over an 11-year period, using large-scale data that spanned all age groups. Utilizing this data will enable surveillance of anti-HAV IgG seroprevalence without additional cost and effort [8].
- The anti-HAV IgG positivity rate encompasses both immunity acquired after HAV infection and vaccine-acquired immunity. On average, several thousand cases of HAV infection are reported annually in the Republic of Korea; therefore, any changes in the anti-HAV IgG positivity rate could be understood as a reflection of the vaccination rate. These findings indicate that it is necessary to implement policies to promote the vaccination of adults, due to the higher risk of severe infections among older individuals and the gender-based difference in the seropositivity rate.
Discussion
- • This study analyzed 1,333,615 cases of hepatitis A antibody testing from January 2012 to June 2022 and found an overall increase in seropositivity rates, including a U-shaped curve that shifted rightward by age.
- • The seropositivity rate diverged by gender in the participants’ early 20s—when men in the Republic of Korea enlist in the military—and the divergence continued subsequently for older individuals.
- • Although the study is limited by a lack of socioeconomic data, its strength is its analysis of large-scale data spanning 11 years across all age groups.
HIGHLIGHTS
Supplementary Material
Table S1.
-
Ethics Approval
The study was approved by the Institutional Review Board of Green Cross Laboratory (no: GCL-2022-1048-01). Informed consent was waived because of the study’s retrospective nature.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: HS, UG, EHL, SGL; Data curation: SA, WP, GC; Formal analysis: HS, WP, GC; Writing–original draft: HS, UG, SA, WP, GC; Writing–review & editing: HS, UG, EHL, SGL. All authors read and approved the final manuscript.
Article information


- 1. Korea Disease Control and Prevention Agency (KDCA). Infectious diseases surveillance yearbook, 2021 [internet]. KDCA; 2022 [Internet]. [cited 2023 Jan 15]. Available from: https://dportal.kdca.go.kr/pot/bbs/BD_selectBbs.do?q_bbsSn=1010&q_bbsDocNo=20220826738398757&q_clsfNo=1. Korean.
- 2. Son H, Lee M, Eun Y, et al. An outbreak of hepatitis A associated with salted clams in Busan, Korea. Epidemiol Health 2022;44:e2022003.ArticlePubMedPDF
- 3. Korea Disease Control and Prevention Agency (KDCA). Hepatitis A virus detection in salted clams imported from China [Internet]. KDCA; 2019 Jul 26 [cited 2021 Aug 9]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=364588&cg_code=&act=view&nPage=1. Korean.
- 4. Korea Disease Control and Prevention Agency (KDCA). Hepatitis A virus detection in salted clams [Internet]. KDCA; 2019 Jun 25 [cited 2021 Sep 22]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=144301&cg_code=&act=view&nPage=1. Korean.
- 5. Lim J, Kim K, Choi S, et al. The effectiveness and limitation of the national childhood hepatitis A vaccination program in the Republic of Korea: findings from the Korean National Health and Nutrition Examination Survey (KNHANES), 2015. PLoS One 2017;12:e0189210.ArticlePubMedPMC
- 6. World Health Organization. WHO position paper on hepatitis A vaccines–October 2022 [Internet]. World Health Organization; 2022 [cited 2022 Dec 23]. Available from: https://www.who.int/publications/i/item/who-wer9740-493-512.
- 7. Lim DH, Sohn W, Jeong JY, et al. The chronological changes in the seroprevalence of anti-hepatitis A virus IgG from 2005 to 2019: experience at four centers in the capital area of South Korea. Medicine (Baltimore) 2022;101:e31639.ArticlePubMedPMC
- 8. Lee DY, Chae SJ, Cho SR, et al. Nationwide seroprevalence of hepatitis A in South Korea from 2009 to 2019. PLoS One 2021;16:e0245162.ArticlePubMedPMC
- 9. Choe YJ, Son H. The changing gender differences in hepatitis A incidence in South Korea. Vaccine 2020;38:712−4.ArticlePubMed
- 10. Im JH, Woo HT, Ha B, et al. Effectiveness of single-dose administration of inactivated hepatitis A virus vaccination in the Republic of Korea armed forces, 2013-2016. J Viral Hepat 2020;27:537−9.ArticlePubMedPDF
- 11. Heo JY, Choe KW, Yoon CG, et al. Vaccination policy in Korean armed forces: current status and future challenge. J Korean Med Sci 2015;30:353−9.ArticlePubMedPMCPDF
- 12. Lemon SM. Type A viral hepatitis: new developments in an old disease. N Engl J Med 1985;313:1059−67.ArticlePubMed
- 13. Ki M, Son H, Choi BY. Causes and countermeasures for repeated outbreaks of hepatitis A among adults in Korea. Epidemiol Health 2019;41:e2019038.ArticlePubMedPMC
References
Figure & Data
References
Citations



 Cite
Cite