Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(4); 2023 > Article
-
Original Article
Prevalence and patterns of adverse events following childhood immunization and the responses of mothers in Ile-Ife, South West Nigeria: a facility-based cross-sectional survey -
Olorunfemi Akinbode Ogundele1
 , Funmito Omolola Fehintola2
, Funmito Omolola Fehintola2 , Mubarak Salami2
, Mubarak Salami2 , Rahmat Usidebhofoh2
, Rahmat Usidebhofoh2 , Mary Aderemi Abaekere2
, Mary Aderemi Abaekere2
-
Osong Public Health and Research Perspectives 2023;14(4):291-299.
DOI: https://doi.org/10.24171/j.phrp.2023.0071
Published online: July 27, 2023
1Department of Community Medicine, University of Medical Sciences, Ondo City, Nigeria
2Department of Community Health, Obafemi Awolowo University, Ile-Ife, Nigeria
- Corresponding author: Olorunfemi Akinbode Ogundele Department of Community Medicine, University of Medical Sciences, Laje Road, PMB 536 Ondo City, Ondo State, Nigeria E-mail: femidele@gmail.com
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 2,229 Views
- 133 Download
Abstract
-
Objectives
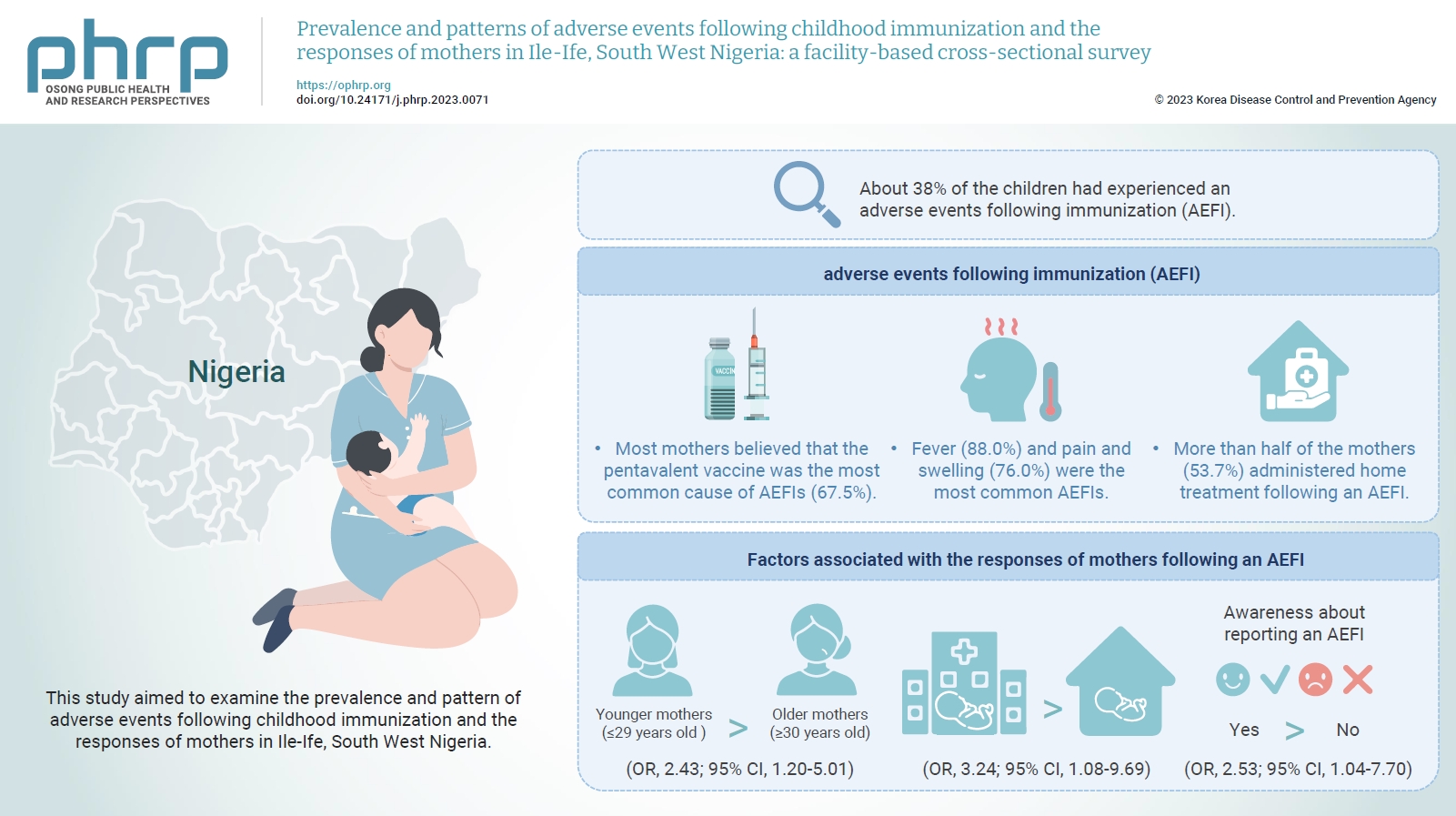
- This study aimed to examine the prevalence and pattern of adverse events following childhood immunization and the responses of mothers in Ile-Ife, South West Nigeria.
-
Methods
- This descriptive cross-sectional study was conducted among 422 mothers of children aged 0 to 24 months attending any of the 3 leading immunization clinics in Ile-Ife, Nigeria. The respondents were selected using the multi-stage sampling technique. Data were collected using a pretested structured interviewer-administered questionnaire and analyzed using IBM SPSS ver. 26.0. The chi-square test was used to test associations, while binary logistic regression was used to determine the predictors of mothers' responses to adverse events following immunization (AEFIs). A p-value of <0.05 was considered statistically significant.
-
Results
- The mean age of the respondents was 29.99±5.74 years. About 38% of the children had experienced an AEFI. Most mothers believed that the pentavalent vaccine was the most common cause of AEFIs (67.5%). Fever (88.0%) and pain and swelling (76.0%) were the most common AEFIs. More than half of the mothers (53.7%) administered home treatment following an AEFI. Younger mothers (odds ratio [OR], 2.43; 95% confidence interval [CI], 1.20–5.01), mothers who delivered their children at a healthcare facility (OR, 3.24; 95% CI, 1.08–9.69), and mothers who were knowledgeable about reporting AEFIs (OR, 2.53; 95% CI, 1.04–7.70) were most likely to respond appropriately to AEFIs.
-
Conclusion
- The proportion of mothers who responded poorly to AEFIs experienced by their children was significant. Therefore, strategies should be implemented to improve mothers’ knowledge about AEFIs to improve their responses.
- Immunization is one of the greatest success stories recorded in modern medicine and one of the most impactful and cost-effective public health strategies ever adopted to protect individuals, families, and the public from infectious diseases and, more recently, from cancers and other chronic diseases [1]. Immunization prevents deaths in all age groups and currently protects 2 to 3 million children yearly from life-threatening childhood diseases [2]. Although childhood immunization has been proven to be objectively beneficial, occasional associated adverse events, which could lead to debilitating conditions that may require hospital admission, are a major source of concern as they may arouse public suspicion and could potentially lead to a loss of immunization gains and heighten vaccine hesitancy. The World Health Organization (WHO) defines adverse events following immunization (AEFIs) as any untoward medical occurrence that follows immunization and that does not necessarily have a causal relationship with vaccine use. The adverse event may be any unfavorable or unintended sign, abnormal laboratory finding, symptom, or disease [3]. AEFIs can be categorized as vaccine product-related reactions, vaccine quality defect-related reactions, immunization error-related reactions (formerly “program errors”), immunization anxiety-related reactions, and coincidental events [3]. In addition, vaccine reactions could be classified as serious or non-serious, with local or systemic manifestations. They could range from fever, irritability, pain at the injection site, skin rash, sores, and difficulty breathing to weakness of the limbs, convulsions, aseptic meningitis, encephalitis, and even death. AEFIs can lead to death or a life-threatening condition that requires hospitalization with or without permanent sequelae [3,4]. AEFIs have been observed globally and have been suggested to be a significant factor contributing to the low vaccination coverage in Nigeria [5].
- The country's current immunization program offers the Bacillus Calmette–Guérin (BCG), polio 3, PENTA3/DPT3, measles, and yellow fever vaccines. The coverage rates are currently suboptimal, with rates of 51.3% for the BCG vaccine, 34% for the polio vaccine, 34.4% for the pentavalent vaccine, 48.1% for the measles vaccine, and 39% for the yellow fever vaccine according to 2021 Multiple Indicator Cluster Survey (MICS) reports [6]. With new vaccines likely to be added to national programs and an increase in the frequency at which vaccines are administered (through, for example, mass immunization campaigns) based on national targets, the immunization schedule will contain more vaccine antigens, possibly resulting in more AEFIs. The MICS report also indicated that 64% of children aged 12 to 23 months did not receive all routine immunizations and that 11% of women refused to vaccinate their children due to their fear of side effects, while 26% did not vaccinate their children due to mistrust and fear [6]. The reported rates of AEFIs in studies vary widely across states in Nigeria, ranging from 19.3% to 57% [7]. Thus, investigation and reporting on AEFIs are critical, given inconsistent rates of AEFIs and reporting about AEFIs across states. However, the Nigerian government, supported by the WHO, has made efforts to strengthen the country’s AEFI surveillance system by forming the National Expert Committee, which is responsible for ensuring that Nigerian health workers can monitor and prevent AEFIs [8]. Regardless of the actual cause, an AEFI may lead to public suspicion about vaccines, resulting in vaccine hesitancy among parents who may refuse further vaccination for their children. This leaves children vulnerable to vaccine-preventable diseases. In addition, it may result in poor vaccination coverage due to mothers who are cautious about vaccination for their children due to their personal experience, the experiences of members of their community, rumors, or distrust about vaccines [9]. AEFIs affect vaccination uptake due to parental concerns about the health of children, as it did in the early 21st century in northern Nigeria when there was widespread parental polio vaccine refusal due to widespread rumors and distrust about the vaccine [9].
- Previous and recent surveys have found wide disparities in the reported rates of AEFIs [7] in different parts of the country, possibly due to contextual differences, socioeconomic inequality, and vaccine-related issues in different geopolitical zones of the country. Therefore, this study aimed to determine the prevalence and prevailing patterns of AEFIs and the responses of mothers in Ile-Ife, South West Nigeria, to fill the contextual gap for evidence-based programming and planning related to AEFIs at the regional and national levels.
Introduction
- Study Area
- This study was conducted in Ile-Ife, Osun, South West Nigeria, between May and July 2022. Ile-Ife consists of 2 local government areas (LGAs; Ife Central and Ife East) and is considered the most populous city in Osun, with a population of 355,281 people, of whom 131,965 are children younger than 5 years old [10]. The LGAs each have major healthcare facilities for administering immunizations. Ife Central, the study site, has 11 wards with 23 public healthcare facilities with a population of 82,240 children under 5 years old as of the time of this study.
- Study Design and Participants
- This was a healthcare facility-based descriptive cross-sectional study conducted with mothers of children aged 0 to 24 months who attended any of the 3 leading immunization clinics in Ile-Ife, Osun, Nigeria. The minimum sample size was estimated using the formula for single proportion [11] and was calculated to be 422 based on an AEFI prevalence of 46.5% [12], an error margin of 5%, and a 95% confidence interval (CI) given a possible 10% non-response rate. The study enrolled mothers with at least 1 eligible child aged 0 to 24 months who had previously completed a full vaccination regimen and mothers who brought their children to 1 of the 3 immunization clinics for the child’s ninth month of vaccinations and who consented to participate in the study. Completion of a full vaccination regimen was confirmed by checking the immunization cards presented by mothers whose children had completed immunization before enrollment in the study. In addition, the ninth month of immunization was used as a reference for enrollment so mothers could easily recall necessary information about previous AEFI experiences following any of the administered vaccines [11]. By the time a child reaches 9 months of age in Nigeria, they are expected to have received all basic immunizations, including BCG, polio 3, PENTA3/DPT3, the measles vaccine, and the yellow fever vaccine, as outlined in the standard vaccination schedule. These immunizations are considered to be an indicator of full coverage, meaning the child would have been exposed to all necessary antigens by this point.
- The index child’ refers to the child chosen for the study about whom the mother provided information for the study. If the mother had more than 1 eligible child, the child whose birthday was nearest to the interview period was selected as the index child.
- Sampling Technique
- As described below, a multi-stage sampling technique was used to select the 422 participants for the study. (1) First stage: Ife Central was randomly chosen from the 2 LGAs in Ile-Ife using simple random sampling by balloting. (2) Second stage: Three major healthcare facilities were purposively chosen among the healthcare facilities in Ife Central since they are major delivery points for immunization services in and around Ile-Ife. These healthcare facilities included the Urban Comprehensive Health Centre (UCHC) at Obafemi Awolowo University Teaching Hospital Complex, Eleyele; the Comprehensive Health Centre (CHC), Enuwa; and the Central Immunization Office (CIO), Aderemi, Ile-Ife. (3) Third stage: A list of mothers with index children who attended the clinic was generated daily in each healthcare facility to form the sampling frame. The participants were then selected from the list using a simple random sampling method by balloting. The number of children registered at UCHC Eleyele averaged 120 per week, while at CHC Enuwa and CIO Aderemi the average was 80 children per week each. Therefore, the sample size proportions allotted to each clinic were 182 from UCHC Eleyele and 120 each from CHC Enuwa and CIO Aderemi. This sampling technique was preferred to give all mothers who met the inclusion criteria an equal chance to be selected. The immunization days at the clinics were Monday through Friday, and the participants were recruited on any of the immunization days. We repeated the process until the targeted number of interviewees was reached.
- Data Collection Instruments
- Data collection was performed using a structured interviewer-administered questionnaire. The questionnaire was designed to meet the study’s objective with multiple questions adapted from the WHO Global Manual on Surveillance of Adverse Events Following Immunization [3] and went through several drafts and reviews. The final draft was pretested with 20 mothers of children aged 0 to 24 months at the immunization clinic of another healthcare facility, and the findings and experiences from the pretest were utilized to modify the instrument. The final tool had 4 sections. The first section covered the participants’ sociodemographic characteristics such as age, sex, education level, occupation, marital status, religion, and others. The second section assessed the participants’ knowledge of childhood vaccines with other related questions. The third section assessed the participants’ knowledge of and awareness about reporting AEFIs using yes or no questions, the occurrence of AEFIs, and the responses of mothers to AEFIs. AEFIs were defined as any untoward medical occurrence that followed immunization and that did not necessarily have a causal relationship with vaccine use that occurred within 28 days after vaccination [3]. The fourth section examined the participants’ perceptions of AEFI.
- Data Analysis
- Data entry and analysis were performed using IBM SPSS ver. 26.0 (IBM Corp.). The descriptive analysis used frequencies and percentage distributions to represent the sociodemographic characteristics of respondents with AEFI patterns presented as bar graphs. In addition, associations between sociodemographic variables, awareness about reporting AEFIs, and the responses of mothers following AEFIs were examined using the Pearson chi-square test with the Fisher exact correction applied where applicable. The outcome variable for the bivariate and multivariate analyses was the response of mothers following an AEFI, which was dichotomized as an “appropriate response” or an “inappropriate response.” An appropriate response in this study was defined as when mothers took the affected child to a healthcare facility within 24 hours of the onset of an AEFI, regardless of initial management at home. In addition, the independent variables, such as awareness about reporting AEFIs, and sociodemographic variables, such as age, marital status, occupation, and others, were recategorized into dichotomous variables for bivariate analyses. Lastly, we conducted binary logistic regression to determine odds ratios (ORs) and 95% CIs to assess the independent effect of selected explanatory variables on the response of mothers following an AEFI. The level of significance was set at p<0.05.
Materials and Methods
- A total of 422 mothers participated in the study, as indicated in Table 1. The number of participants in their 20s (45.5%) was comparable to the number of participants in their 30s (46.9%). The mean age was 29.99±5.74 years. Nearly all of the participants were married (98.6%), and 52.4% had a tertiary education. The majority of the participants were of Yoruba descent from South West Nigeria (85.3%). A large proportion (93.4%) attended antenatal clinics and delivered their children at healthcare facilities (89.6%). Over two-thirds (68.2%) had 1 to 2 children, and most of the index children were boys (52.6%). A high proportion of the study respondents (90.8%) were employed.
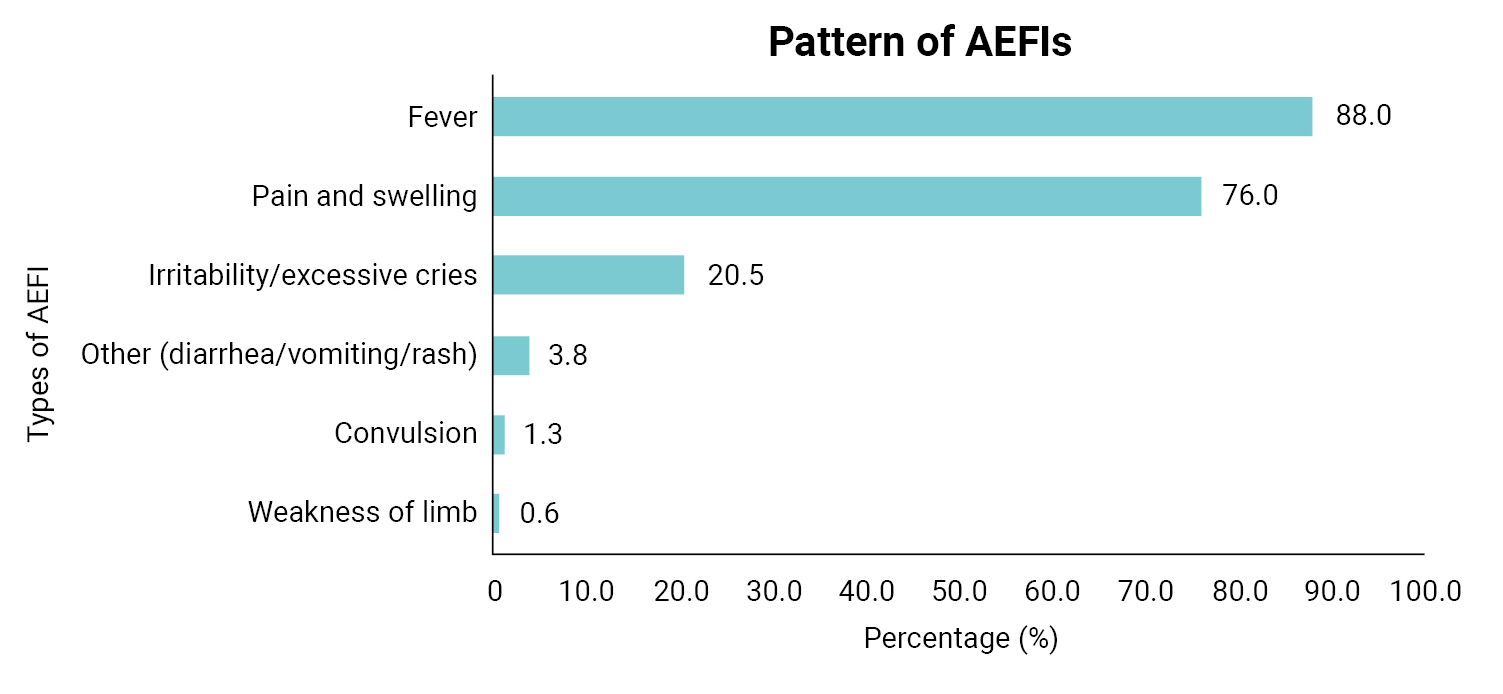
- Overall, 37.9% of the index children had experienced an AEFI (Table 2). The majority of the AEFIs reported by mothers in the study were generalized/systemic in presentation (58.6%) and mainly occurred within 6 hours of vaccination (63.8%). In addition, according to the mothers, the pentavalent vaccine was believed to be the most prevalent cause of AEFIs (67.5%), followed by the pneumococcal conjugate vaccine (65.6%) and the BCG vaccine (26.2%). Fever (88.0%) and pain and swelling (76.0%) were the most common AEFIs experienced (Figure 1). Irritability and excessive crying comprised 20.5% of AEFIs, while rare and more serious vaccine reactions such as seizures were less frequent (1.3%) among the AEFIs reported by mothers. The responses of mothers following the development of AEFIs in their children varied, with most mothers (53.7%) treating the child at home using paracetamol, tepid sponging, or other methods, while 26.3% reported that they went to a hospital within 24 hours of the onset of symptoms and 20.0% that they went to a hospital more than 24 hours after symptom onset (Figure 2).
- As a result of bivariate analysis, factors such as the sex of the child, birth order, and ethnicity had p-values of ≥0.20 [11] and were not included in the logistic regression. However, as a result of binary logistic regression, maternal age, place of delivery, and awareness about reporting AEFIs remained significant determinants of the response of mothers following an AEFI. Younger mothers were twice as likely to respond appropriately following an AEFI than older mothers (OR, 2.43; 95% CI, 1.20–5.01). In addition, mothers who delivered in healthcare facilities were 3 times more likely to respond appropriately to AEFIs than those who delivered at home (OR, 3.24; 95% CI, 1.08–9.69). Awareness about reporting AEFIs was also significantly associated with responding appropriately following an AEFI. The odds of responding appropriately to an AEFI were 2.5 times higher (OR, 2.53; 95% CI, 1.04–7.70) among mothers who were aware of reporting AEFIs (Table 3).
Results
- This study examined the prevalence and pattern of AEFIs and the response of mothers in Ile-Ife, South West Nigeria. With the introduction of new vaccines to the national immunization schedule, more frequent vaccinations to meet national targets, and exposure to more vaccine antigens, there is an increased likelihood of AEFIs. Therefore, the availability of information on background rates and patterns of reported AEFIs will help address AEFI-related concerns.
- In this study, about 37.9% of children were reported by mothers to have experienced an AEFI in some form. This rate was consistently lower than the rates reported in North Central [12], North West [13], and South South [14] Nigeria, although it was slightly higher than the rate reported in an earlier study in North West Nigeria in 2016 [15]. The consistently different rates across regions, including the rate reported in this study, may be a result of contextual differences in study settings, differences in the education levels of mothers between regions as documented in past studies [12–15], coincidental events that may have been reported as AEFIs by mothers in some of the studies due to a close temporal association with the timing of immunization, different vaccine handling and storage techniques, differences in the manufacturers of vaccines across zones, and, notably, ethnic and genetic differences between children, given that many studies from different zones of the country employed a similar cross-sectional research design. There may also be differences among mothers in their level of awareness about reporting AEFIs across zones, which is also closely linked to the education level of mothers.
- This study found a generalized/systemic presentation to be the most common site of AEFIs reported by mothers and the pentavalent vaccine to be the vaccine most often believed to be the cause of AEFIs. The finding regarding generalized presentation is consistent with earlier studies [13,15–17]. The finding that the pentavalent vaccine was the most frequently cited cause of an AEFI is also not unexpected since it is a combination of 5 vaccines in a single dose and has been reported to be associated with increased incidence of AEFIs and due to the diphtheria, pertussis, and tetanus (DPT) components of the pentavalent vaccine [13,18,19]. We found fever to be the most common AEFI observed by mothers following the vaccination of their children. This finding is similar to the results of many previous Nigerian [12–15] and international [20,21] studies that found fever to be the most common AEFI. However, the rate reported in our study was slightly higher than that in other studies, with 8 in 10 vaccinated children experiencing fever. This finding is not surprising since most of the respondents involved in this study received the pentavalent vaccine. Moreover, previous studies that compared the DPT vaccine and the pentavalent vaccine found fever to be the most common complication of the DPT vaccine, which is a component of the pentavalent vaccine [22]. Likewise, considering the antigenic nature of vaccines introduced into the body, the normal physiologic reaction of the body can cause fever due to the production of cytokines.
- Regarding the responses of mothers to AEFIs, approximately 5 in 10 mothers managed the AEFI at home using simple treatments such as paracetamol and tepid sponging. However, in addition to initial treatment at home, it would have been appropriate for the mothers to report the AEFI to a healthcare facility within 24 hours to receive a health worker’s assessment and reassurance, which only about a quarter of the mothers in this study did. Reporting at healthcare facilities is important not only to the child but also for the health system to record AEFIs following all childhood immunizations in order to respond appropriately due to vaccine safety concerns and issues with handling and monitoring vaccines. The findings regarding the appropriateness of the mothers’ responses align with those of a study of mothers in Jos, North Central Nigeria [12], although we found a higher level than was reported in the previous study. This finding contrasts with the results of a study of mothers from Benin City, South South Nigeria [14], where almost all (99%) of the respondents reported AEFIs to a healthcare facility; however, the study did not measure the time since the onset of symptoms when classifying the responses of mothers unlike in our study. The timing of mothers’ responses is, however, essential for effectively managing the response to AEFIs among both mothers and children since it offers an opportunity to calm the anxiety of mothers and provide care for the child. The contrasting findings of this study provide further evidence for contextual differences in AEFI-related issues across the country’s regions.
- In our study, the mothers’ ages, place of delivery, and awareness about reporting AEFIs were significantly associated with the appropriateness of mothers’ responses to AEFIs. The finding that younger mothers were more likely to report AEFIs than older mothers in this study could be due to past experiences with AEFIs among older mothers, who may have been less anxious about AEFIs due to a previous positive or negative experience, unlike younger mothers who may not have had any experience with AEFIs and thus responded to their child’s symptoms with more urgency. This finding suggests that experience with childcare impacts mothers’ childcare practices related to care-seeking and their reaction to their child’s illness. We also found that mothers who delivered at healthcare facilities were more likely to report AEFIs, possibly because they were previously informed about immunization by health workers and may have had the opportunity to be educated before or during the child's first vaccination, which is often administered before discharge after delivery. Antenatal clinic attendance did not show any significant association with the appropriateness of mothers’ responses to AEFIs, possibly because information about AEFIs are not sufficiently covered in antenatal health education. In addition, some women who attend antenatal clinics end up not delivering in healthcare facilities and miss the opportunity to receive information on AEFIs when their children are vaccinated. Lastly, awareness about AEFIs was significantly associated with the appropriateness of mothers’ responses when reporting AEFIs in this study. This may not be surprising since knowledge improves children’s health practices and care-seeking behaviors, as established in previous studies [14,23], and other studies that reported that education impacts vaccination completion [24,25] give support to the finding in this study that awareness improves AEFI reporting, as similarly suggested in an earlier study [14]. Based on the findings presented above, there is a need to strengthen the country's AEFI surveillance system further by devising and implementing AEFI educational programs targeting pregnant women at antenatal clinics for mothers during postnatal visits and at immunization clinics. This is essential since it would enable health workers to better gather data directly from mothers. In addition, delivery at healthcare institutions should be encouraged since it provides mothers with an opportunity to receive vital information about childcare. It is worth noting that a significantly higher percentage of mothers in our study were employed compared to the national average of women in the workforce [26]. However, we found no significant association between occupation and the appropriateness of the mothers’ responses to AEFIs. This result may be due to the purposive sampling used to determine our study location. This finding has implications for future research, which may yield different findings from this study.
- The study's strength is that it provides insight into the prevalence of AEFIs in the South West region of Nigeria, given the wide disparities in the rates of AEFIs reported in other regions, and suggest the need for a national survey to determine the prevalence of AEFIs and identify the various factors associated with reporting AEFIs among mothers for effective planning and programming related to AEFIs at both the national and regional levels.
- Although this study provides valuable insights, it also has multiple important limitations. First, we were unable to determine the incidence of AEFIs in response to specific vaccines since the number of children who received each vaccine was not recorded. Second, data on the occurrence of AEFIs in the index children were self-reported by mothers, which may have resulted in the over- or under-reporting of AEFIs in the study due to possible recall bias and the inability to verify the data due to the nature of the study design. A cohort study design is suggested in the future to determine the incidence of AEFIs rather than the cross-sectional design used in the current study. Third, the experiences of mothers often vary widely based on the ages of their children, although this was minimized by including children at the ninth month of vaccinations to ensure similarity. Lastly, the cross-sectional nature of the data restricted the ability to infer causal relationships.
Discussion
- Our study indicates that a significant proportion of mothers (approximately one-third) reported AEFIs among their children. However, our findings also highlighted a general lack of appropriate responses from mothers in such situations. Awareness of AEFIs and the place of delivery, among other factors, were strongly related to the appropriateness of the mothers’ responses. To improve mothers’ awareness of AEFIs and strengthen their responses to AEFIs, we recommend the implementation of targeted educational programs for mothers about AEFI-related issues.
Conclusion
- • A high prevalence of adverse events following immunization (AEFI) among respondents was found.
- • The appropriateness of mothers’ responses to AEFIs was also found to be poor.
- • Mothers’ awareness about reporting AEFIs and delivery at a healthcare facility predicted an appropriate response to AEFIs.
HIGHLIGHTS
-
Ethics Approval
The study protocol was approved by the Ethical Committee of the Institute of Public Health, Obafemi Awolowo University, Ile-Ife (No: IPH/OAU/12/1991). Informed consent was obtained from the respondents before the interview, and the data were anonymized. The confidentiality of the respondents was assured and maintained during and after the study.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: OAO, FOF; Data curation: OAO, FOF; Formal analysis: OAO; Investigation: MS, RU, MAA; Methodology: all authors; Project administration: FOF; Resources: FOF, MS, RU, MAA; Software: OAO; Supervision: OAO, FOF; Validation: OAO, FOF, MS, RU, MAA; Writing–original draft: OAO; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Article information

| Variable | Frequency (%) |
|---|---|
| Child’s experience of an AEFI (n=422) | |
| Experienced | 160 (37.9) |
| Not experienced | 262 (62.1) |
| AEFI by site reported (n=160)a) | |
| Local | 123 (41.4) |
| Systemic/generalized | 174 (58.6) |
| Time until the onset of AEFIs (h) | |
| <6 | 102 (63.8) |
| 6–12 | 40 (25.0) |
| 13–23 | 11 (6.9) |
| ≥24 | 7 (4.4) |
| Vaccines linked to AEFIsa) | |
| Bacillus Calmette–Guérin | 42 (26.2) |
| Pentavalent vaccine | 108 (67.5) |
| Pneumococcal conjugate vaccine | 105 (65.6) |
| Oral polio vaccine | 36 (22.5) |
| Inactivated polio vaccine | 39 (24.3) |
| Measles vaccine | 8 (5.0) |
| Yellow fever vaccine | 1 (0.6) |
| Variable | Appropriate response | Inappropriate response | OR (95% CI) | p |
|---|---|---|---|---|
| Mother’s age (y) | ||||
| ≤29 | 23 (37.1) | 39 (62.9) | 2.43 (1.20–5.01) | 0.013 |
| ≥30 | 19 (19.4) | 79 (80.6) | 1 | |
| Occupation | ||||
| Employed | 35 (24.5) | 108 (75.5) | 0.46 (0.17–1.27) | 0.152 |
| Unemployed | 7 (41.2) | 10 (58.8) | 1 | |
| Marital status | ||||
| Not married | 6 (42.9) | 8 (57.1) | 1 | |
| Married | 36 (24.7) | 110 (75.3) | 2.29 (0.07–6.79) | 0.139 |
| Education level | ||||
| Primary education or lower | 1 (20.0) | 4 (80.0) | 1 | |
| Secondary education or above | 41 (26.5) | 114 (73.5) | 0.70 (0.09–5.10) | P=0.206a) |
| Antenatal clinic attendance | ||||
| Yes | 37 (25.0) | 111 (75.0) | 0.47 (0.15–1.49) | 0.303 |
| No | 5 (41.7) | 7 (58.3) | 1 | |
| Place of delivery | ||||
| Healthcare facility | 38 (30.2) | 88 (69.8) | 3.24 (1.08–9.69) | P=0.030a) |
| Home | 4 (11.8) | 30 (88.2) | 1 | |
| Awareness about reporting an AEFI | ||||
| Yes | 35 (31.3) | 77 (68.8) | 2.53 (1.04–7.70) | 0.032 |
| No | 7 (14.6) | 41 (85.4) | 1 |
- 1. World Health Organization (WHO). Immunization [Internet]. WHO; 2019 [cited 2022 Dec 8]. Available from: https://www.who.int/news-room/facts-in-pictures/detail/immunization.
- 2. United Nation Emergency Fund (UNICEF). Immunization: vaccines are the world's safest method to protect children from life-threatening diseases [Internet]. UNICEF; 2021 [cited 2022 Dec 8]. Available from: https://www.unicef.org/immunization.
- 3. World Health Organization (WHO). Global manual on surveillance of adverse events following immunization [Internet]. WHO; 2016 [cited 2022 Dec 8]. Available from: https://www.who.int/publications/i/item/10665206144.
- 4. Al Awaidy S, Bawikar S, Prakash KR, et al. Surveillance of adverse events following immunization: 10 years' experience in Oman. East Mediterr Health J 2010;16:475−80.
- 5. Akwataghibe NN, Ogunsola EA, Broerse JE, et al. Exploring factors influencing immunization utilization in Nigeria: a mixed methods study. Front Public Health 2019;7:392. ArticlePubMedPMC
- 6. National Bureau of Statistics (NBS) and United Nations Children’s Fund (UNICEF). Multiple indicator cluster survey (MICS) 2016-17: survey findings report [Internet]. NBS and UNICEF; 2017 [cited 2022 Dec 30]. Available from: https://www.unicef.org/nigeria/media/1406/file/Nigeria-MICS-2016-17.pdf.
- 7. Mohammed LA, Aliyu AA, Maiha BB, et al. Knowledge, perception and reporting attitude of adverse effects following immunization among primary healthcare workers in Sabon Gari local government area Zaria, Kaduna State, Nigeria. Niger J Basic Clin Sci 2018;15:81−6.Article
- 8. World Health Organization (WHO).. Scale-up measures to reduce adverse events and increase immunization uptake in Nigeria [Internet]. WHO; 2018 [cited 2022 Dec 30]. Available from: https://www.afro.who.int/news/scale-measures-reduce-adverse-events-and-increase-immunization-uptake-nigeria.
- 9. Ogundele OA, Ogundele T, Beloved O. Vaccine hesitancy in Nigeria: contributing factors–way forward. Niger J Gen Pract 2020;18:1−4.Article
- 10. National Population Commission. 2006 population and housing census of the Federal Republic of Nigeria: priority tables. National Population Commission; 2009.
- 11. Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. John Wiley & Sons; 2000.
- 12. Afolaranmi TO, Hassan ZI, Sodipo OY, et al. Knowledge of adverse events following immunization, its prevalence and actions of mothers of children aged 0-23 months in a tertiary health institution in Jos, North Central Nigeria. J Med Trop 2020;22:57−64.ArticlePubMedPMC
- 13. Maizare HI, Tsiga-Ahmed FI, Jibo AM, et al. Prevalence and patterns of adverse events following immunisation among children less than 24 months attending immunisation clinics in Kano, Nigeria. Ann Afr Med Res 2021;4:149. ArticlePDF
- 14. Adam VY, Onowugbeda ED, Osuji OI, et al. Prevalence and management of perceived adverse events following immunisation in infants attending Well Baby Clinics in Benin City, Nigeria. J Comm Med Prim Health Care 2020;32:57−67.
- 15. Lawan UM, Amole GT, Wali NY, et al. Pattern of adverse events following immunization in nourished and malnourished infants in Kano, North-Western Nigeria. Sahel Med J 2016;19:131−6.Article
- 16. Baranski K, Gajda M, Braczkowska B, et al. Parental declaration of adverse event following immunization in a cross-sectional study in Poland. Int J Environ Res Public Health 2019;16:4038. ArticlePubMedPMC
- 17. Tsafack M, Ateudjieu J. Improving community based AEFI (adverse events following immunization) reporting rate through telephone "beep" in a Cameroon health district: a randomized field trial. Pan Afr Med J 2015;22:351. ArticlePubMedPMC
- 18. Harris T, Nair J, Fediurek J, et al. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012-15. Vaccine 2017;35:2600−4.ArticlePubMed
- 19. Santos MC, Pontes Netto VB, Andrade MS. Prevalence and factors associated with the occurrence of adverse events following immunization in children. Acta Paul Enferm 2016;9:626−32.
- 20. Ekrami Noghabi M, Saffar MJ, Rezai S, et al. Immunogenicity and complications of the pentavalent vaccine in Iranian children. Front Pediatr 2021;9:716779. ArticlePubMedPMC
- 21. Izadi S, Mohammadi M, Sartipi M, et al. Acute adverse events following immunization with DTP-HB-Hib pentavalent vaccine in the first year of life. East Mediterr Health J 2023;29:6−14.ArticlePubMed
- 22. Khazaei Z, Moradi G, Zahraei SM, et al. The comparison of the adverse events of pentavalent vaccine and DPT vaccine in 2-6 months infants in Iran: a national study. Ann Glob Health 2020;86:11. ArticlePubMedPMC
- 23. Bbaale E. Factors influencing childhood immunization in Uganda. J Health Popul Nutr 2013;31:118−29.ArticlePubMedPMC
- 24. Adedokun ST, Uthman OA, Adekanmbi VT, et al. Incomplete childhood immunization in Nigeria: a multilevel analysis of individual and contextual factors. BMC Public Health 2017;17:236. ArticlePubMedPMCPDF
- 25. Ogundele OA, Ogundele T, Fehintola FO, et al. Determinants of incomplete vaccination among children 12-23 months in Nigeria: an analysis of a national sample. Tzu Chi Med J 2022;34:448−55.ArticlePubMedPMC
- 26. International Labour Organization. Labor force participation rate, female (% of female population ages 15+) (modeled ILO estimate)–Nigeria [Internet]. World Bank Group; 2023 [cited 2023 May 29]. Available from: https://data.worldbank.org/indicator/SL.TLF.CACT.FE.ZS?locations=NG.
References
Figure & Data
References
Citations




 Cite
Cite