Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(3); 2023 > Article
-
Original Article
Effectiveness of the COVID-19 vaccine in the Honam region of the Republic of Korea -
In-Sook Shin1,2
 , Yong-Pyo Lee2
, Yong-Pyo Lee2 , Seung-Hoon Lee2
, Seung-Hoon Lee2 , Jae-Young Lee3
, Jae-Young Lee3 , Jong-Ha Park2
, Jong-Ha Park2 , Yoon-Seok Chung2
, Yoon-Seok Chung2
-
Osong Public Health and Research Perspectives 2023;14(3):197-206.
DOI: https://doi.org/10.24171/j.phrp.2022.0308
Published online: June 8, 2023
1Division of Control for Zoonotic and Vector Borne Disease, Korea Diseases Control and Prevention Agency, Cheongju, Republic of Korea
2Division of Infectious Disease Diagnosis Control, Honam Regional Center for Disease Control and Prevention, Korea Diseases Control and Prevention Agency, Gwangju, Republic of Korea
3Division of Vaccine-Preventable Diseases Control and National Immunization Program, Korea Diseases Control and Prevention Agency, Cheongju, Republic of Korea
- Corresponding author: Yoon-Seok Chung Division of Infectious Disease Diagnosis Control, Honam Regional Center for Disease Control and Prevention, Korea Diseases Control and Prevention Agency, 103 Sangmusimin-ro, Seo-gu, Gwangju 61947, Republic of Korea E-mail: Rollstone93@korea.kr
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 1,869 Views
- 63 Download
Abstract
-
Objectives
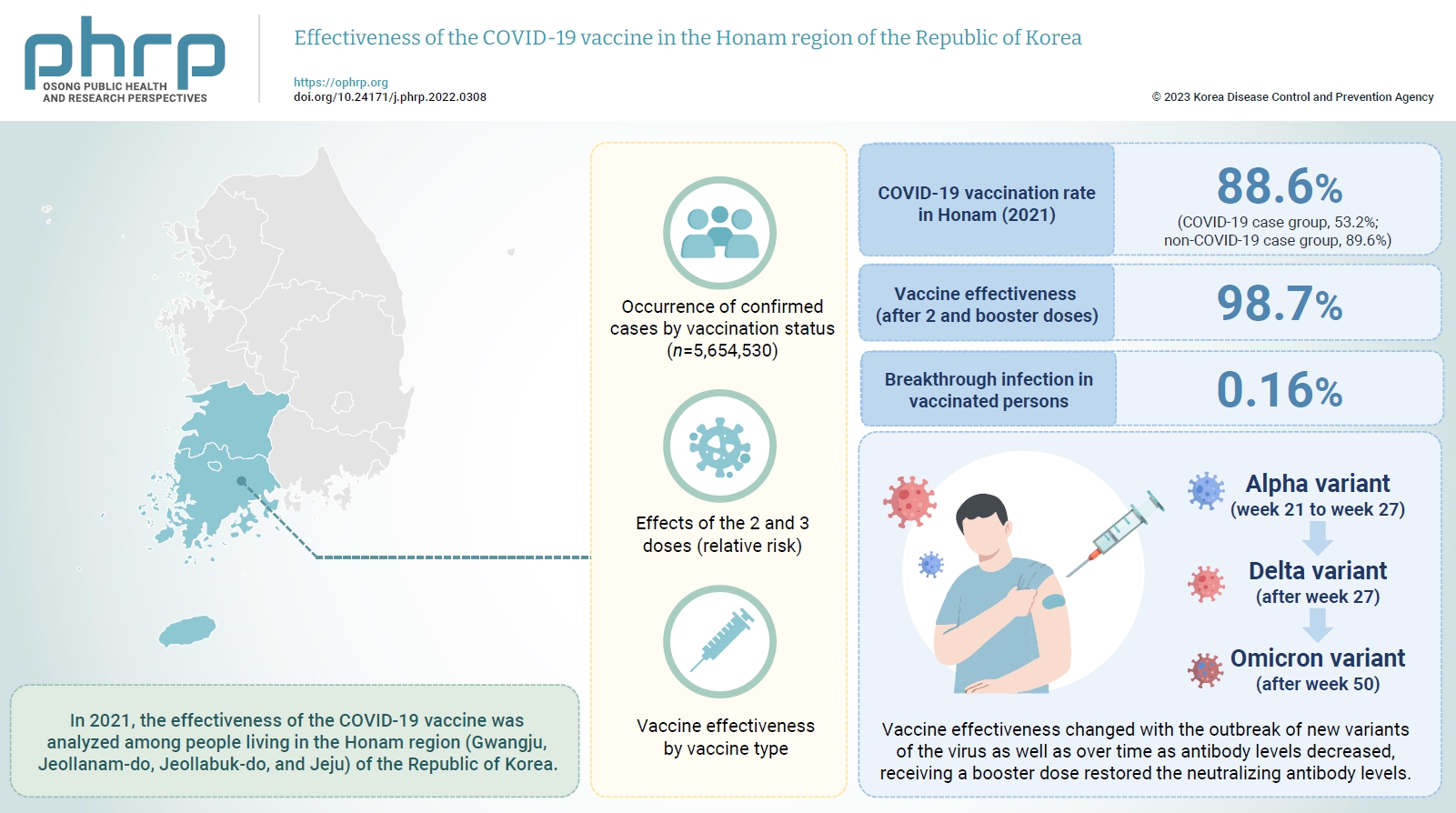
- In 2021, the effectiveness of the COVID-19 vaccine was analyzed among people living in the Honam region (Gwangju, Jeollanam-do, Jeollabuk-do, and Jeju) of the Republic of Korea. And we investigated changes in the dominant virus strain.
-
Methods
- This study used the data provided by the Korean Ministry of the Interior and Safety for individuals ≥12 years old in the Honam region, and the Integrated Disease and Health Management System of the Korea Centers for Disease Control and Prevention for COVID-19-vaccinated individuals as of December 31, 2021. Statistical analyzes were performed using IBM SPSS ver. 23.0. The occurrence of confirmed cases by vaccination status, the relative risk, and vaccine effectiveness by vaccine type were calculated.
-
Results
- In 2021, the COVID-19 vaccination rate in Honam was 88.6%. The overall vaccine effectiveness (after 2 and 3 doses) was 98.7% (p<0.001). and the breakthrough infection rate was 0.16%. From week 21 to week 27 of 2021 (June 27 to July 3), the genome sequencing results were mostly alpha variants. The Delta variant emerged as the dominant variant after 27 weeks and the Omicron variant was found at 50 weeks (December 5–11).
-
Conclusion
- Vaccine effectiveness changed with the outbreak of new variants of the virus as well as over time as antibody levels decreased. that the prevention effectiveness of vaccination in Honam was >98%, and the effect among persons who received 2 doses was >90% regardless of the vaccine type. Although vaccine effectiveness decreased because of reduced antibody levels over time (as observed in breakthrough infections), receiving a booster dose restored the neutralizing antibody levels.
- The first coronavirus disease 2019 (COVID-19) case in the Republic of Korea was confirmed on January 20, 2020, in a Chinese woman (aged 35 years) from Wuhan, China who traveled to the Republic of Korea through the Incheon International Airport. The crisis alert level in the Republic of Korea was upgraded from “attention” to “caution.” As 4 additional cases were confirmed, the level was elevated to “alert” on January 27, 2020. Approximately 120,000 cases had been confirmed worldwide in nearly 110 countries by January 31 of the same year, and the World Health Organization (WHO) declared the COVID-19 outbreak a global public health crisis [1].
- As the number of cases of COVID-19 soared worldwide, the total number of imported and domestically confirmed cases exceeded 500 in the Republic of Korea. On February 23, 2020, the country upgraded the crisis alert level to “severe” and transitioned to an aggressive response system. With the continued spread of infection worldwide, the WHO declared COVID-19 a global pandemic on March 11, 2020. The pandemic is ongoing due to the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants.
- A vaccine is an important tool to suppress outbreaks, prevent the spread of infection, and reduce the number of deaths. The COVID-19 vaccine was first administered in the United Kingdom (UK) on December 8, 2020. In the Republic of Korea, vaccination programs began on February 26, 2021, following the approval of COVID-19 vaccines developed by Oxford AstraZeneca (henceforth A vaccine; February 10, 2021), Pfizer BioNTech (C vaccine; March 5, 2021), Janssen (D vaccine; April 7, 2021), and Moderna (B vaccine; May 21, 2021) by the Korea Ministry of Foods and Drug Safety. The features of each vaccine are shown in Table 1 [2].
- In 2021, 4 COVID-19 vaccines were available in the Republic of Korea, 2 viral vector vaccines (A and D) and 2 mRNA vaccines (C and B). In mRNA vaccines, an intermediate carrier is injected that links the DNA and proteins during protein synthesis based on the DNA containing genetic information and helps human cells to produce an extrinsic protein. Viral vector vaccines deliver a modified virus (vector) to human cells to create antigens and induce an immune response [3].
- The Republic of Korea imported a total of 194 million vaccine doses (as of October 25, 2021), including 20 million each from COVID-19 Vaccines Global Access (COVAX) and Oxford AstraZeneca, 67 million from Pfizer BioNTech, 7 million from Janssen, and 40 million each from Moderna and Novavax. The vaccines were administered stepwise to the Republic of Korean nationals as well as foreigners, including illegal immigrants. As of March 30, 2022, 44,482,876 individuals (86.7% of the Republic of Korean population) had received 2 vaccine doses, while 32,688,629 (69.4%) individuals had received the third dose. However, the number of confirmed cases increased in March 2022 by >20 times compared with that in December 2021 (from 1,217 to 24,739 per 100,000 population).
- No study has yet examined the effectiveness of the COVID-19 vaccine for an entire regional population in the Republic of Korea. Therefore, the current study aimed to analyze the vaccine effectiveness in the Honam region, which has a high proportion of older adults. This region, located south of the Geum River, is comprised of both the mainland landmass and islands, includes both cities and rural areas, and encompasses Gwangju, Jeollanam-do, Jeollabuk-do, and Jeju.
- In this study, the data of persons living in Honam and vaccinated for COVID-19 between January 1, 2021, and December 31, 2021, were retrospectively analyzed (a retrospective cohort study) to investigate vaccine effectiveness by the number of doses and vaccine type. Additionally, the whole genome sequencing results of positive specimens were analyzed to examine changes in the dominant variants. To assess the pandemic in the Republic of Korea, the scale and pattern of confirmed cases were identified based on the type of dominant COVID-19 variant.
Introduction
- This retrospective cohort study used data provided by the Korea Ministry of the Interior and Safety of people aged ≥12 years who lived in the Honam region and who were vaccinated, according to the regional vaccination registration system of the Integrated Disease & Health Management System of the Korea Disease Control and Prevention Agency (KDCA), as of December 31, 2021.
- The database of vaccinated persons aged ≥12 years in the Honam region who were registered in the Integrated Disease & Health Management System from January 1 to December 31, 2021 was matched with the database of those with confirmed COVID-19 who were registered in the KDCA Honam Center for Disease Response to identify the COVID-19 case and non-COVID-19 case groups. Unvaccinated persons were identified by subtracting the subset of all vaccinated persons from the Honam population aged ≥12 years. Unvaccinated individuals were classified into the COVID-19 case or non-COVID-19 case groups by matching with the database of individuals confirmed to have COVID-19 in Honam.
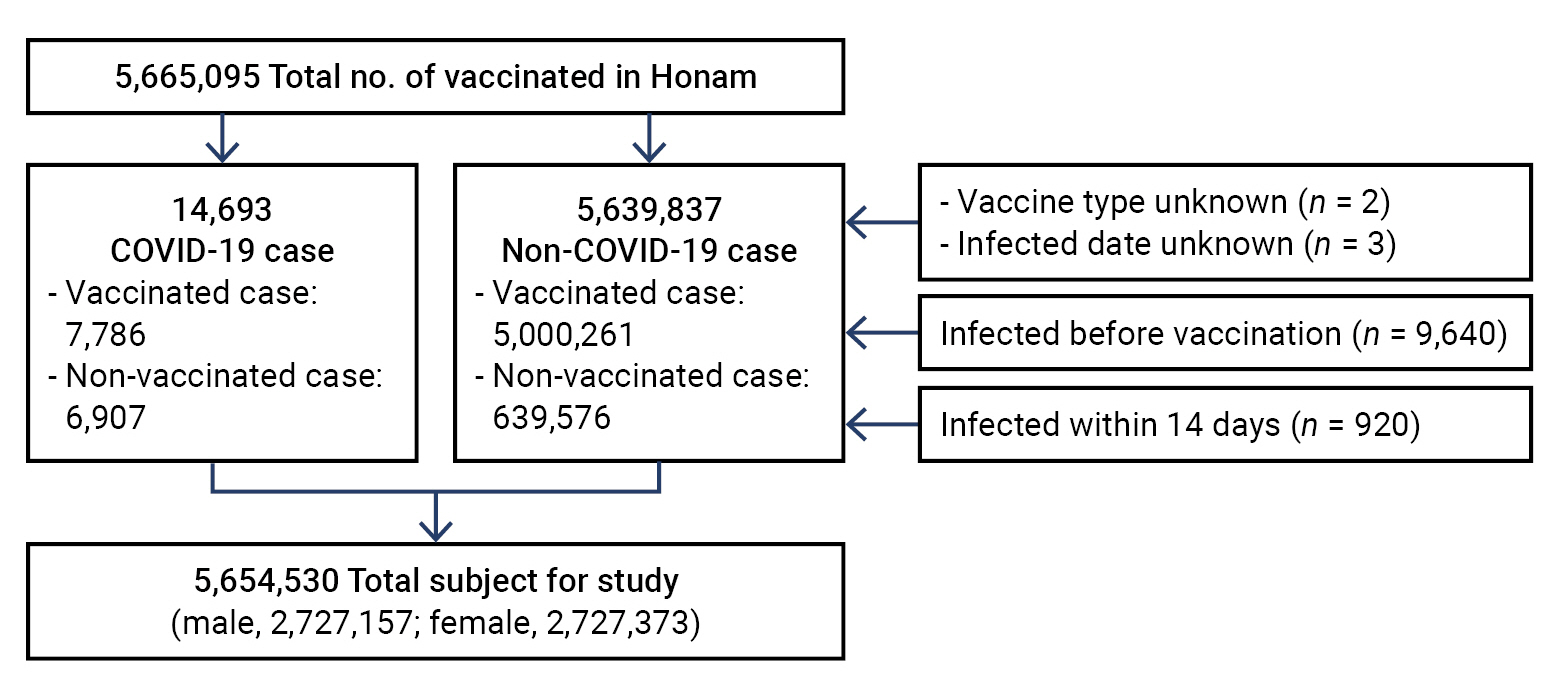
- The Honam region has a total population of 5,665,095 individuals, excluding children aged <12 years. Of the total population, 2,732,563 were men and 2,932,532 were women, with a sex ratio of 48.2:51.8. Of the vaccinated persons, 3 who received the COVID-19 vaccine abroad and 2 whose date of infection onset was unknown were excluded from the analysis of vaccine effectiveness as it was impossible to identify the type of vaccine (in the former) and timing of infection (in the latter). Additionally, 9,640 persons who were infected before vaccination and 920 who were infected within 14 days after vaccination were excluded because their infections were independent of the vaccine.
- A total of 10,565 individuals aged ≥12 years were excluded from the total population of 5,665,095 individuals. Hence, 5,654,530 individuals were included in the final analysis, with 14,693 individuals included in the case group (7,786 vaccinated and 6,907 unvaccinated) and 5,639,837 in the non-case group (5,000,261 vaccinated and 639,576 unvaccinated) (Figure 1).
- The effects of the second and third doses were examined by assessing the relative risk (RR) in the total population of 5,654,530 persons, and differences in the incidence of infection according to vaccine type were evaluated by estimating the median survival time and by performing a log-rank test using the data of 5,842 persons who received 2 doses. In persons who received the second or third dose, the rate of breakthrough infection was computed by age group, and the period in which each of the dominant variants was detected via whole genome sequencing, and the volume of confirmed cases was examined.
- Statistical Analyses
- Statistical analyses were performed using IBM SPSS ver. 23.0 (IBM Corp.). The chi-square test was used to determine the proportion of patients in the case group by vaccination status, while survival analysis (Kaplan-Meier, log-rank test) was performed to evaluate the temporal difference in the incidence of infection according to vaccine type. The attack rate in the vaccinated and unvaccinated persons was computed by vaccine type to assess the RR and to estimate the vaccine effectiveness by vaccine type.
- Ethics Statement
- The data were collected as part of the public health response to the COVID-19 pandemic. This paper was approved by the Institutional Review Board (IRB) of the KDCA for bioethics examination (IRB No: 2022-11-06-PE-A; 2022-11-28). The informed consent was waived because of the retrospective nature of this study.
Materials and Methods
- As of December 31, 2021, of 5,654,530 persons (the Honam population aged ≥12 years, after excluding persons with missing data on infection occurrence, those infected before vaccination, and those infected within 14 days following vaccination from the total population of 5,665,095 individuals), 14,693 were confirmed to have COVID-19. Thus, the overall infection rate was 0.13% and the sex ratio was 51:49, with COVID-19 more prevalent in men. Among the age groups, individuals aged 60–69 years showed the highest rate of confirmed cases (33.3%), followed by those aged 20–29, 30–39, 40–49, and 12–19 years.
- Approximately 5,008,047 persons were vaccinated, with a total vaccination rate of 88.6%. The vaccination rates were 53.2% in the COVID-19 case group and 89.6% in the non-COVID-19 case group. More than half of the vaccinated individuals received mRNA vaccines (53.4%), while 27.75% received V-mRNA vaccines (cross-vaccination with viral vector and mRNA vaccines) and 7.35% received virus vector vaccines.
- Regarding the number of vaccine doses, 435,662 individuals received a single dose, 2,373,517 received 2 doses, and 2,198,868 received 3 doses. Most individuals who received a single dose had to wait to be eligible for the second dose. The rate of confirmed cases according to the number of vaccine doses was 0.42% for a single dose, 0.25% for 2 doses, and 0.01% for 3 doses. Thus, the rate of confirmed cases decreased as the number of doses increased.
- Among individuals who received a single dose, the rate of confirmed cases was highest (7.9%) in those who received the A vaccine, followed by those who received the D, B, and C vaccines. Among individuals who received 2 doses, the rate of confirmed cases was the highest (0.7%) in those who received the AA vaccines, followed by those who received the CC, AC, BB, and BC vaccines. Among individuals who received 3 doses, the rate of confirmed cases was <0.0% in all vaccine combinations.
- Overall, the breakthrough infection rate in vaccinated persons was 0.16% (7,786 of 4,791,820). The rate among those who received 2 doses was 0.12% (5,848 of 4,791,820).
- Analysis of the timing of post-vaccination COVID-19 by vaccine type was performed by investigating those who received 2 doses (standard vaccination protocol). Overall, the post-vaccination infection rate was highest (25.25%) at 3 to 4 months after the second dose. By vaccine type, the rate was highest at 1 to 2 months after BB vaccination, at 2 to 3 months after CC vaccination, at 3 to 4 months after AA vaccination, and at 4 to 5 months after AC vaccination.
- In the Republic of Korea, COVID-19 vaccination was initiated on February 26, 2021. In the first quarter, A and C vaccines were administered to convalescent hospital/facility inpatients, residents aged <65 years, non-healthcare employees, and employees of healthcare institutions classified as hospitals including infectious disease hospitals. In the second quarter, the A, C, and D vaccines were administered to individuals aged 60 to 74 years, patients with chronic illness, all healthcare institution and pharmacy employees, essential social service personnel, convalescent hospital/facility inpatients, residents aged ≥65 years, individuals aged ≥75 years, and military personnel. In the third quarter, the C, B, and D vaccines were administered to unvaccinated persons in previously targeted groups, high school seniors, individuals aged ≥18 years, and foreigners. In the fourth quarter, the C and B vaccines were administered to pregnant women, persons eligible for the third dose, and children aged 12 to 17 years (Table 2).
- The incidence of COVID-19 was 1.07% in unvaccinated persons and 0.16% in vaccinated persons, and the RR was 0.145. The risk of infection was significantly reduced with vaccination (p<0.05, RR<1) (Table 3).
- Persons who were confirmed to have COVID-19 before receiving the second dose (6 cases infected after vaccination with BC) and those infected <14 days following the second dose were excluded from the analysis because the infection was unrelated to the second dose. Additionally, persons who were administered the BBB, BBC, CCB, or BCC vaccines were excluded because none of them was confirmed to have COVID-19; therefore, it was not possible to assess the effectiveness of those vaccine combinations.
- The attack rate was calculated in persons with confirmed COVID-19 after receiving the second dose out of those who received 2 doses of the COVID-19 vaccine (including cross-vaccination) and in persons with confirmed COVID-19 after receiving the booster vaccinations out of those who had received booster vaccinations. The number of patients with confirmed COVID-19 after receiving the second dose was 5,842, which included those with confirmed disease ≥14 days after receiving the second dose and those with confirmed disease <14 days following the administration of the booster vaccinations. A total of 126 persons had confirmed COVID-19 ≥14 days after receiving the booster vaccinations. The overall attack rate was 0.14%, and 5,968 individuals were confirmed to have COVID-19 after the second dose and booster vaccinations.
- The overall effectiveness of the COVID-19 vaccine (2 and 3 doses) was 98.7%. The vaccine effectiveness in persons who received more than 2 doses was 99.7%. In persons who received the second dose, excluding those who received the AA vaccines, the vaccination effectiveness was >97.4% (Table 4).
- We examined the incidence of confirmed cases by vaccine type (viral vector, mRNA, and V-mRNA vaccines). The proportion of confirmed cases was 0.87% in persons who received a viral vector vaccine, 0.12% in those who received an mRNA vaccine, and 0.03% in those who were cross-vaccinated (p<0.001) (Table 5).
- Survival analysis was performed on the data of individuals in the COVID-19 case group who had received 2 doses, that is, those who received the second dose at least 14 days earlier and those who received the third dose <14 days before developing COVID-19 (n=5,842). Individuals with confirmed COVID-19 who received a single dose and who developed COVID-19 <14 days after receiving the second dose were excluded from the analysis.
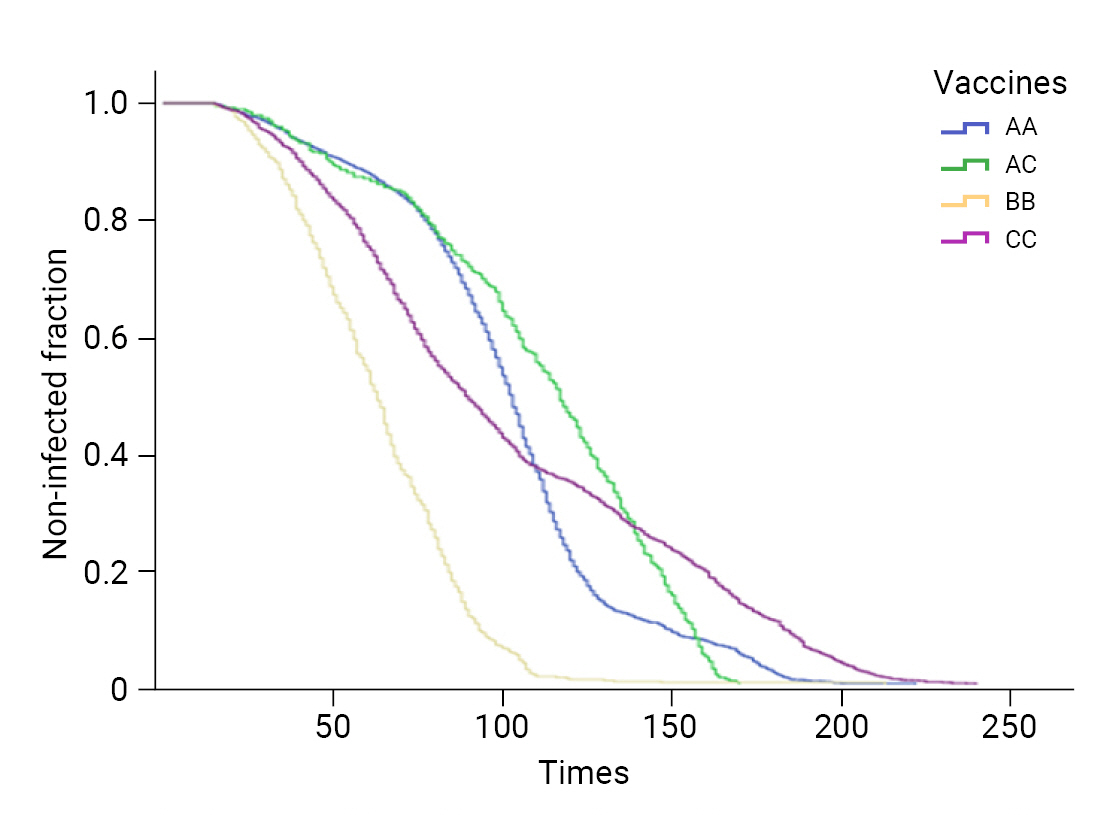
- The overall median survival time was 98.3 days (95% confidence interval [CI], 97.1–99.4 days). The median survival time was longest in individuals vaccinated with AC (110.2 days) and shortest in those vaccinated with BB (63.2 days) (Table 6).
- Using the log-rank test, survival analysis of the data from the COVID-19 cases who received 2 doses showed that the difference between AC and CC was not significant, although the differences in other factors were significant (p<0.05) (Table 7; Figure 2).
- Of all vaccinated persons in Honam (n=5,008,047), 7,786 had confirmed COVID-19 at least 14 days after vaccination, and the breakthrough infection rate was 0.16%. The breakthrough infection rates by age group were 0.23% in individuals aged 60 to 69 years and 0.13% each in those aged 20 to 39 and 40 to 59 years. The rate was lowest (0.05%) in individuals aged 12 to 19 years (Table 8).
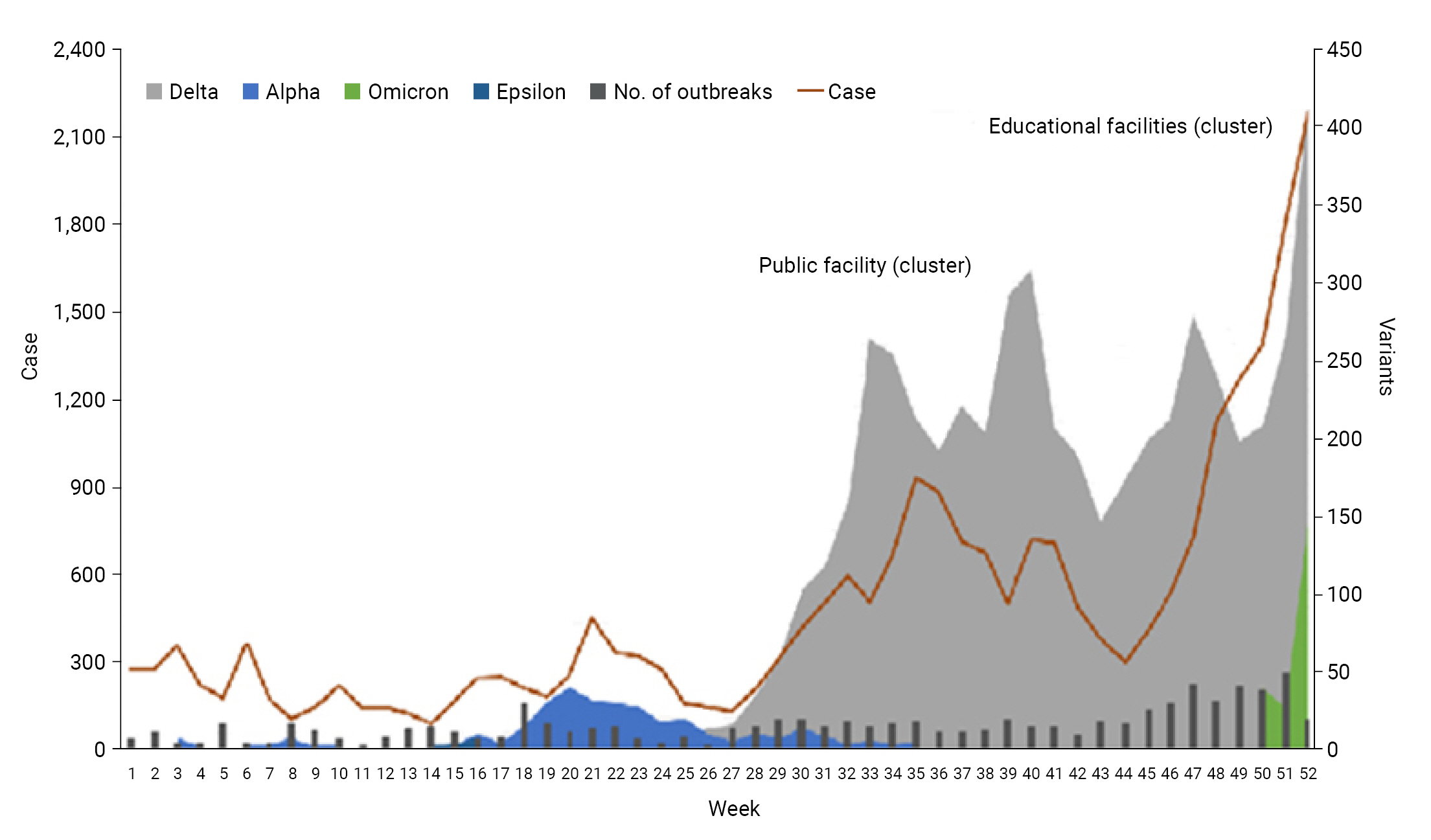
- The periods in which the SARS-CoV-2 variants were found in Honam and the scale of the outbreaks were examined. During the period of week 21 to week 27 (June 27–July 3), the average number of weekly confirmed COVID-19 cases was 170, and the virus identified via whole genome sequencing was the Alpha variant in most cases.
- After week 27, Delta emerged as the dominant variant, and the average weekly number of confirmed COVID-19 cases in Honam increased to 426 from July through October. In week 50 (December 5–11), the Omicron variant was detected in specimens, and the average weekly number in weeks 50 to 53 (December 26–January 1) sharply increased to 1,788 (Figure 3).
Results
- As of December 2021, the COVID-19 vaccination rates in Honam were 84.0% for those who received 2 doses and 41.1% for those who received 3 doses. These rates were higher by 1.2%p and 6.7%p, respectively, than the corresponding rates reported nationwide [4].
- The overall effectiveness of vaccination in Honam among all persons vaccinated in 2021, except for confirmed COVID-19 cases unrelated to vaccination, was 98.7%. The vaccine effectiveness rates by vaccine type were 91.3% for viral vector vaccines, 98.8% for mRNA vaccines, and 99.7% for V-mRNA vaccines. In confirmed cases of COVID-19, 0.87% received a viral vector vaccine, 0.12% received an mRNA vaccine, 0.03% were cross-vaccinated, and a significant difference was observed according to vaccine type (p<0.001).
- In persons who received 2 doses, the vaccine effectiveness was lowest for AA (93.4%). The prevention effectiveness was 98.5%, 97.4%, and 99.4% in individuals who received AC cross-vaccination, CC vaccination, and BB vaccination, respectively. Thus, in all vaccine types, the prevention effectiveness was >93%.
- Countries such as Israel, the United States (US), and the UK reported on the prevention effectiveness of the 2 COVID-19 mRNA vaccines (developed by Pfizer and Moderna). In Israel, the analysis of approximately 4,710,000 persons who received 2 doses of the Pfizer vaccine showed a 97.2% (95% CI, 96.8%–97.5%) effectiveness in the prevention of hospital admission and a 96.7% (95% CI, 96.0%–97.3%) effectiveness in the prevention of death [5]. In a previous US study, the prevention effectiveness of 2 doses of the Pfizer or Moderna vaccines was 90% in healthcare professionals, and the prevention of hospital admission in persons aged ≥65 years was 94% (95% CI, 49%–99%) [6]. In the UK, the COVID-19 prevention effectiveness of 2 doses of the Pfizer vaccine among healthcare professionals was 85% (95% CI, 74%–96%) [7]. In Honam, which encompasses both mainland landmass and islands, as well as urban and rural areas, and has an age distribution skewed toward older age groups, the prevention effectiveness of the COVID-19 vaccine was higher than that reported in other countries. However, the prevention effectiveness of a vaccine also depends on various factors, such as the intensity of an outbreak and the level of response. Therefore, the vaccine effectiveness in Honam may change if the period of the Omicron outbreak is included in the analysis.
- Breakthrough infection refers to the occurrence of infection >14 days after vaccination. It occurs when the antibodies in vaccines become ineffective because: (1) the individual’s immunity level and physical condition are compromised, (2) the antibody levels have decreased in the body over time following vaccination, and (3) a mismatched antigenic determinant has evolved with the emergence of viral variants. The overall breakthrough infection rate was 0.16%, and this rate was highest in persons aged ≥60 years (0.23%). The breakthrough infection rate was higher in this age group than in other age groups, because persons aged 60 to 79 years were prioritized in the early phase of vaccination and the effect of their vaccines had decreased over time.
- As the COVID-19 pandemic continued, several variants emerged. The WHO monitors variants by classifying them into variants of interest and variants of concern according to transmissibility, mortality rate, and the extent of changes in the virus versus the existing vaccines, diagnosis capabilities, and treatments. In Honam, the first confirmed case was identified on January 30, 2020, and the ratio of confirmed cases remained quite low until July 2021. Most cases were infected with the Alpha variant, which was classified as a variant of concern, and the average weekly number of confirmed cases was 170 until week 27.
- After week 27, Delta emerged as the dominant variant, and the third outbreak occurred in the Republic of Korea. The average weekly number of confirmed cases in Honam from July to October increased to 426. The transmissibility of the Delta variant was 1.6 times higher than that of the Alpha variant and resulted in a sharp rise in the number of confirmed cases, transmitted in public facilities such as karaoke businesses and saunas. In Scotland, the effectiveness of the vaccine against the Delta variant was 87.9% in persons who received 2 doses of the Pfizer vaccine and 59.8% in persons who received 2 doses of the AstraZeneca vaccine [6]. It was reported in the Morbidity and Mortality Weekly Report of the US Centers for Disease Control and Prevention that an mRNA-based vaccine was 91% effective before the Delta variant outbreak, but the effectiveness decreased to 66% when Delta became the dominant variant. According to that report, the effectiveness of the vaccine against the variants gradually decreased, although statistical uncertainty existed since the estimation was based on a relatively short study period [8]. Nonetheless, the report stressed that vaccination was of paramount importance, even at a 66% reduction in infection risk.
- The fourth outbreak in the Republic of Korea occurred in week 45 (October 31–November 6) in educational institutions such as schools and after-school academies, and the average weekly number of confirmed cases in Honam increased in November and December to 1,249, which was 3 times higher than was reported during the third outbreak. The Omicron variant, first identified in week 50, was less likely to cause severe illness, but showed higher transmissibility than the Delta variant; thus, the number of cases increased rapidly [9,10].
- In the analysis of 1,861 confirmed cases during week 52 (December 19–25), out of 611 specimens (32.8%) the Delta variant was identified in 73.3% (n=488) of the cases and Omicron in 26.7% (n=163). The latter had rapidly increased within 2 weeks, up from 13.9% in week 50.
- The KDCA Division of Novel Pathogen Analysis investigated the risk of reinfection with a variant as well as vaccine effectiveness by performing a plaque reduction neutralization test [3]. The potency of neutralizing the G genotype virus and its variants (Alpha and Beta) was analyzed using the convalescent sera of persons with COVID-19 and those infected with the Alpha or Beta variant, as well as the sera of vaccinated persons. The antibodies in already infected or vaccinated persons maintained the potency for neutralizing the Alpha and Beta variants, although the potency was somewhat lower in the latter [11]. According to a study by the KDCA National Institute of Health in which neutralizing antibody levels were compared by variant, the effectiveness of preventing infection by the Omicron variant decreased over time following the second dose, although the effectiveness of preventing severe illness was maintained. After administration of the third dose, the level of neutralizing antibodies increased, indicating that the potency for neutralizing Omicron and Delta variants increased. In persons who were vaccinated with the C or A vaccine for the first 2 doses and received their third dose with the C vaccine, the level of neutralizing antibodies against Omicron increased 10.5 to 113.2 times within 2 to 4 weeks when compared with levels before receiving the third dose. This suggested the need for an additional dose.
- The WHO (January 7, 2022) emphasized that the risk of Omicron infection was “very high,” since the scope of hazards could expand due to the rapid transmission and high likelihood of large-scale outbreaks within a short period of time. On January 27, 2022, the European Center for Disease Prevention and Control stressed the importance of vaccination by classifying countries with a high vaccine penetration as “high” risk and countries with a low vaccine penetration as “very high” risk.
- The COVID-19 pandemic persists due to the emergence of new variants. However, throughout human history, vaccines have played a significant role in effectively suppressing many infectious diseases; the COVID-19 vaccine also suppressed the spread of infection and reduced the occurrence of severe cases. Nevertheless, high expectations and anxiety regarding vaccines coexist. The complete safety and efficacy of vaccines for all individuals cannot be guaranteed, and the COVID-19 vaccine is not an exception. Moreover, vaccine effectiveness does not remain constant, as antibody levels decrease over time following each vaccination. However, an analysis of vaccinated persons found that in the Republic of Korea, the incidence of infection was lower in vaccinated persons and the effectiveness of the vaccine was >95%. Although the vaccine effectiveness changed as antibody levels decreased over time, a booster dose could restore the neutralizing antibody levels.
- In this study, the prevention effectiveness of the vaccines was evaluated based on data in the Honam region, which has complex geographical characteristics (a mix of urban and rural areas) and a high proportion of older adults. The dominant variants were examined in each of the outbreaks in which the number of confirmed cases increased despite the high vaccine rate. This study had some limitations. The confirmed cases after vaccination were not classified according to severity and the number of deaths was not identified. This analysis did not cover the longer duration of the Omicron outbreak; thus, the estimation of the vaccine’s prevention effectiveness was likely affected. In addition, although it was plausible for the level of viral exposure to vary in different outbreak situations, the analysis was conducted assuming a constant exposure level. This assumption may have impacted the results of our evaluation of the various vaccines.
- Further studies should be conducted in the Republic of Korea to examine the effectiveness of vaccines according to the severity of COVID-19, the death and survival rates, and the variant type. The immunological characteristics of the specific variants involved should also be investigated. The current study reflects the epidemiological features of COVID-19 in the Republic of Korea and can serve as baseline data to promptly respond during future outbreaks of a rapidly evolving virus.
Discussion
- In 2021, the COVID-19 vaccination rate in Honam was 88.6%. the prevention effectiveness of vaccination was >98%, and the effect among persons who received 2 doses was >90% regardless of the vaccine type. The genome sequencing results were mostly alpha variants. The Delta variant emerged as the dominant variant after 27 weeks and the Omicron variant was found at 50 weeks.
HIGHLIGHTS
-
Ethics Approval
This study was approved by the IRB (IRB No: 2022-11-06-PE-A; 2022-11-28) of the KDCA for bioethics examination. The informed consent was waived because of the retrospective nature of this study.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: ISS, JHP; Data curation: SHL, YPL; Formal analysis: ISS, JYL; Investigation: ISS; Methodology: ISS, SHL, YPL; Project administration: YSC, ISS; Software: ISS; Supervision: JHP, YSC; Validation: ISS, SHL, YPL, JYL; Visualization: ISS; Writing–original draft: ISS; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Article information
| Characteristic | Total | COVID-19 case | Non-COVID-19 case |
|---|---|---|---|
| Total | 5,654,530 | 14,693 | 5,639,837 |
| Sex | |||
| Male | 2,727,157 (48.2) | 7,445 (50.7) | 2,719,713 (48.2) |
| Female | 2,927,373 (51.8) | 7,248 (49.3) | 2,920,125 (51.8) |
| Age (y) | |||
| 12–19 | 472,806 (8.4) | 1,315 (8.9) | 471,491 (8.4) |
| 20–29 | 701,706 (12.4) | 2,326 (15.8) | 699,380 (12.4) |
| 30–39 | 651,383 (11.5) | 2,226 (15.2) | 649,157 (11.5) |
| 40–49 | 880,249 (15.6) | 2,069 (14.1) | 878,180 (15.6) |
| 50–59 | 951,304 (16.8) | 1,870 (12.7) | 949,434 (16.8) |
| ≥60 | 1,997,082 (35.3) | 4,887 (33.3) | 1,992,195 (35.3) |
| Vaccination status | |||
| Vaccinated | 5,008,047 (88.6) | 7,786 (53.0) | 5,000,261 (88.7) |
| Unvaccinated | 646,483 (11.4) | 6,907 (47.0) | 639,576 (11.3) |
| Vaccine platform | |||
| Viral | 415,765 (7.4) | 3,617 (24.6) | 412,148 (7.3) |
| mRNA | 3,023,058 (53.5) | 3,687 (25.1) | 3,019,371 (53.5) |
| Viral-mRNA | 1,569,224 (27.8) | 482 (3.3) | 1,568,742 (27.8) |
| Unvaccinated | 646,483 (11.4) | 6,907 (47.0) | 639,576 (11.3) |
| Breakthrough infectiona) by age group (y) | |||
| 12–19 | 386,800 (6.8) | 185 (1.3) | 386,615 (6.9) |
| 20–29 | 688,798 (12.2) | 695 (4.7) | 688,103 (12.2) |
| 30–39 | 604,061 (10.7) | 961 (6.5) | 603,100 (10.7) |
| 40–49 | 825,252 (14.6) | 1,128 (7.7) | 824,124 (14.6) |
| 50–59 | 930,963 (16.5) | 1,178 (8.0) | 929,785 (16.5) |
| ≥60 | 1,572,173 (27.8) | 3,639 (24.8) | 1,568,534 (27.8) |
| Unvaccinated | 646,483 (11.4) | 6,907 (47.0) | 639,576 (11.3) |
| Breakthrough infectiona) by vaccine type | |||
| AA | 379,675 (6.7) | 2,678 (18,2) | 376,997 (6.7) |
| BB | 884,634 (15.6) | 570 (3.9) | 884,064 (15.7) |
| CC | 851,569 (15.1) | 2,200 (15.0) | 849,369 (15.1) |
| AC | 243,244 (4.3) | 394 (2.7) | 242,850 (4.3) |
| BC | 14,395 (0.3) | 6 (0.0) | 14,389 (0.3) |
| 1 Dose & booster | 3,014,205 (46.6) | 4,616 (13.2) | 3,009,589 (46.7) |
| Unvaccinated | 646,483 (11.4) | 6,907 (47.0) | 639,576 (11.3) |
| Vaccination status | COVID-19 case | Non-COVID-19 case | Total | RR (95% CI) | p |
|---|---|---|---|---|---|
| Vaccinated | 7,786 | 5,000,261 | 5,008,047 | 0.145 (0.137–0.153) | 0.001* |
| Unvaccinated | 6,907 | 639,576 | 646,483 |
| Vaccine | Type | COVID-19 case | Non-COVID-19 case | Total | Vaccinated attack rate (VE %) | p |
|---|---|---|---|---|---|---|
| 2 Doses | AA | 2,678 | 376,997 | 379,675 | 0.71 (93.40) | <0.001* |
| BB | 570 | 884,064 | 884,634 | 0.06 (99.40) | ||
| CC | 2,200 | 849,369 | 851,569 | 0.26 (97.40) | ||
| AC | 394 | 242,850 | 243,244 | 0.16 (98.48) | ||
| DB | 16 | 108,216 | 108,232 | 0.01 (99.86) | ||
| DC | 4 | 16,637 | 16,641 | 0.02 (99.78) | ||
| Booster | AAB | 9 | 691,047 | 691,056 | 0.00 (99.99) | |
| AAC | 52 | 332,233 | 332,285 | 0.02 (99.85) | ||
| ACC | 7 | 176,658 | 176,665 | 0.00 (99.96) | ||
| CCC | 38 | 657,724 | 657,762 | 0.01 (99.95) | ||
| Total | 5,968 | 4,335,795 | 4,341,763 | 0.14 (98.71) | ||
| Platform | COVID-19 case | Non-COVID-19 case | Total | RR (95% CI) | p- |
|---|---|---|---|---|---|
| Viral vector | 3,617 | 412,148 | 415,765 | 0.81 (0.781–0.846) | <0.001* |
| mRNA | 3,687 | 3,019,371 | 3,023,058 | 0.12 (0.107–0.118) | |
| Viral-mRNA | 482 | 1,568,742 | 1,569,224 | 0.03 (0.026–0.032) |
- 1. World Health Organization (WHO). International health regulations (2005), 3rd ed [Internet]. WHO; 2016 [cited 2020 Feb 12]. Available from: https://www.who.int/ihr/publications/9789241580496/en/.
- 2. Ministry of Food and Drug Safety (MFDS). Characteristics and Principles of Operation of COVID-19 Vaccine [Internet]. MFDS; 2021 [cited 2021 Jul 1]. Available from: https://www.mfds.go.kr/brd/m_99/view.do?seq=44935&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1. Korean.
- 3. Kang HM, Choi EH, Kim YJ. Updates on coronavirus disease-2019 vaccine and consideration in children. Pediatr Infect Vaccine 2021;28:7−20.ArticlePDF
- 4. Korea Disease Control and Prevention Agency (KDCA). COVID-19 vaccination and domestic outbreak status Press release [Internet]. KDCA; 2021 [cited 2021 Dec 31]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=718152&cg_code=C01&act=view&nPage=96. Korean.
- 5. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819−29.ArticlePubMedPMC
- 6. Ahn SH, Lee SH. Updates on coronavirus disease 19 vaccine and its clinical application. Korean J Fam Pract 2021;11:236−46.Article
- 7. Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 2021;397:1725−35.PubMedPMC
- 8. Fowlkes A, Gaglani M, Groover K, et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance: eight U.S. locations, December 2020-August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1167−9.ArticlePubMedPMC
- 9. Lee JJ, Choe YJ, Jeong H, et al. Importation and transmission of SARS-CoV-2 B.1.1.529 (Omicron) variant of concern in Korea, November 2021. J Korean Med Sci 2021;36:e346.ArticlePubMedPMCPDF
- 10. Ministry of Health NZ. Delta Response Rapid Review [Internet]. Ministry of Health NZ; 2022 [cited 2022 Jun 20]. Available from: https://www.health.govt.nz/publication/delta-response-rapid-review.
- 11. Oh SJ, Lee E, Lee J, et al. Analysis of COVID-19 mutation virus neutralization using serum of patients with complete cure and vaccination. Public Health Wkly Rep 2021;14:2149−50.
References
Figure & Data
References
Citations

- Figure
- Related articles
-
- Risk factors for transmission in a COVID-19 cluster infection in a high school in the Republic of Korea
- Vaccine effectiveness and the epidemiological characteristics of a COVID-19 outbreak in a tertiary hospital in Republic of Korea
- Risk factors for COVID-19 outbreaks in livestock slaughtering and processing facilities in Republic of Korea
- Risk factors for deaths associated with COVID-19 according to the cause of death classification in Republic of Korea
- Early countermeasures to COVID-19 at long-term care facilities in Gwangju Metropolitan City, Republic of Korea


 Cite
Cite