Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(2); 2023 > Article
-
Original Article
The incidence and clinical characteristics of myocarditis and pericarditis following mRNA-based COVID-19 vaccination in Republic of Korea adolescents from July 2021 to September 2022 -
Ju-Young Sim1
 , Seung-Yun Kim2
, Seung-Yun Kim2 , Eun-Kyoung Kim3
, Eun-Kyoung Kim3
-
Osong Public Health and Research Perspectives 2023;14(2):76-88.
DOI: https://doi.org/10.24171/j.phrp.2023.0032
Published online: April 18, 2023
1Division of Healthcare Associated Infection Control, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
2Adverse Event Investigation Team, COVID-19 Vaccination Task Force, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
3Division of Infectious Disease Control, Bureau of Infectious Disease Policy, Korea Disease Contrㅊol and Prevention Agency, Cheongju, Republic of Korea
- Corresponding author: Eun-Kyoung Kim Division of Infectious Disease Control, Bureau of Infectious Disease Policy, Korea Disease Control and Prevention Agency, 187 Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju 28159, Republic of Korea E-mail: eis5548@korea.kr
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- Age-specific information regarding myocarditis/pericarditis in adolescents following mRNA-based coronavirus disease 2019 (COVID-19) vaccination in Asia remains insufficient. This study investigated the incidence and clinical characteristics of myocarditis/pericarditis in Republic of Korea adolescents after mRNA-based COVID-19 vaccination.
-
Methods
- This retrospective descriptive study utilized patient data from the Korea Immunization Management System. Incidence rates were calculated according to age and sex. Clinical characteristics (symptoms/signs, laboratory values, and imaging results) were compared between mild and severe cases.
-
Results
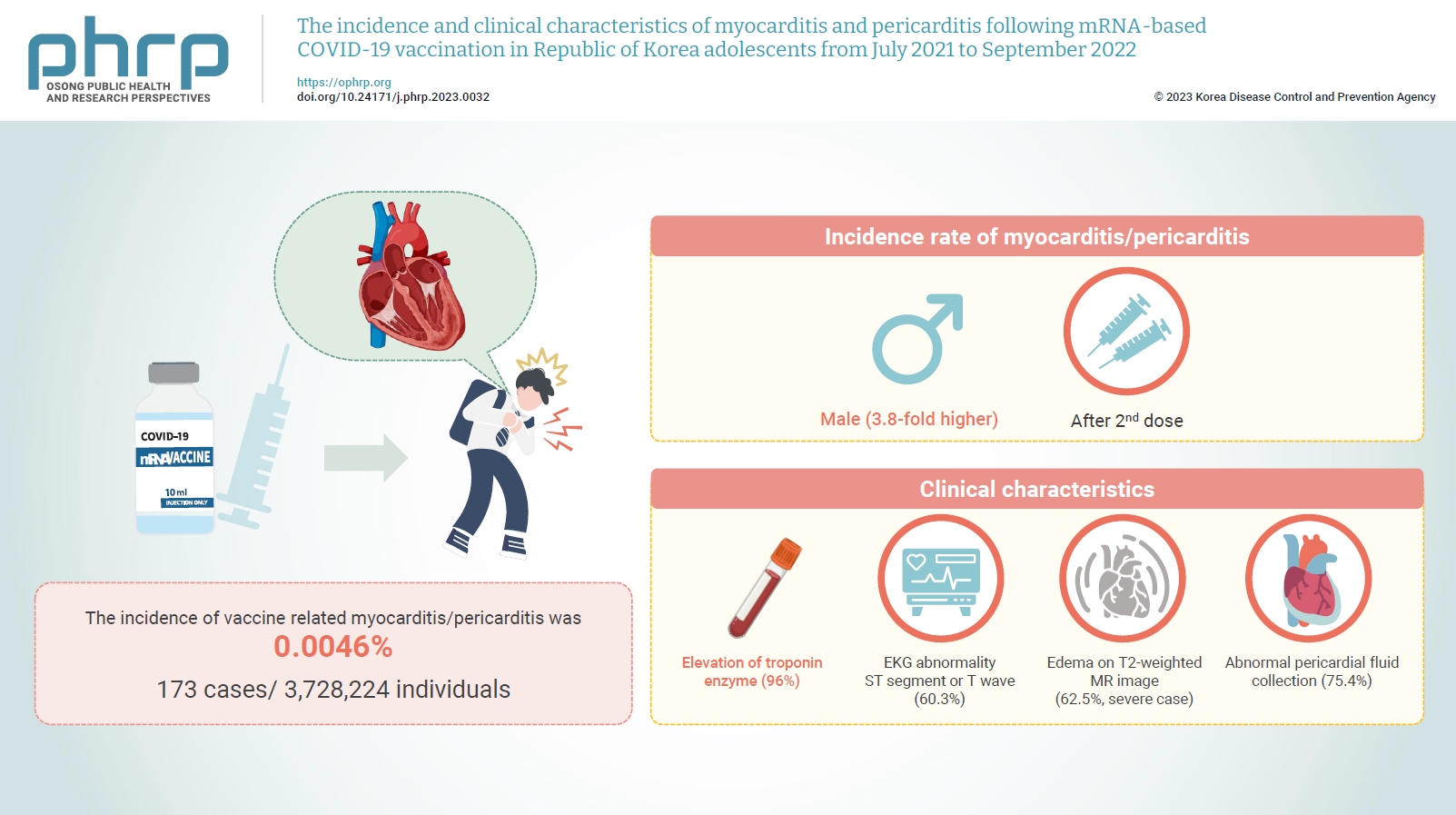
- Between July 19, 2021 and September 30, 2022, 3,728,224 individuals aged 12 to 19 years received 6,484,165 mRNA-based COVID-19 vaccines, and 173 cases met the case definition for myocarditis/pericarditis: 151 mild (87.3%) and 22 severe (12.7%). The incidence was 3.8-fold higher in males than in females. Troponin I/troponin T was elevated in 96% of myocarditis cases, demonstrating higher sensitivity than creatine kinase-myocardial band (67.6%) or C-reactive protein (75.2%). ST-segment or Twave on electrography abnormalities were found in 60.3% (85/141). Paroxysmal/sustained atrial/ventricular arrhythmias were more common in severe than in mild cases (45.5% vs. 16.8%, p=0.008). Edema on T2-weighted magnetic imaging occurred in 21.6% (8/37) and 62.5% (5/8) of mild and severe cases, respectively (p=0.03). Abnormal pericardial fluid collection or pericardial inflammation was found in 75.4% of pericarditis cases (49/65).
-
Conclusion
- Myocarditis/pericarditis occurred in rare cases following mRNA-based COVID-19 vaccination. Most cases were mild, but the incidence was higher in adolescent males and after the second dose. As bivalent severe acute respiratory syndrome coronavirus 2 mRNA vaccination started in South Korea in October 2022, the post-vaccination incidence of myocarditis/pericarditis should be closely monitored, considering clinical characteristics.
- Coronavirus disease 2019 (COVID-19) symptoms in children are generally mild. However, serious complications, including multisystem inflammatory syndrome in children, can occur [1,2]. To prevent COVID-19 infection and minimize the occurrence of serious complications, vaccination has been introduced alongside non-pharmacological approaches, such as enforcing mask policies, social distancing, and school closures [3]. By reducing the requirement for quarantine and the number of hospital admissions due to COVID-19, vaccination has had additional sociopsychological benefits, such as decreasing school absences and limiting the mental health issues associated with temporary shutdowns [4].
- In South Korea, the mRNA-based BNT162b2-BioNTech COVID-19 vaccine was first made available to high school seniors and high school employees on July 19, 2021. Vaccination was then extended to adolescents aged 16 to 17 years on October 18, 2021, and to those aged 12 to 15 years on November 1, 2021 [5–7].
- Although the COVID-19 vaccines are effective in preventing the development of severe symptoms and death from COVID-19 infection [8–10], they have also been reported to cause myocarditis and pericarditis in rare cases [11–15]. In a review of the relationship between mRNA-based COVID-19 vaccines and myocarditis, the Advisory Committee on Immunization Practices reported that myocarditis or pericarditis occurred more frequently in male adolescents and young adults after the second dose [15]. In addition, several reports related to the occurrence and characteristics of myocarditis and pericarditis following mRNA-based COVID-19 vaccination have been published in multiple countries [14,16–19]. One systematic review analyzed the clinical presentation and outcomes of 74 patients who developed myocarditis after administration of mRNA vaccines [20]. Meanwhile, in South Korea, after the COVID-19 vaccination plan for high school seniors was announced, a study was conducted to examine the epidemiology and clinical characteristics of myocarditis and pericarditis in patients aged 17 years and younger prior to the introduction of COVID-19 vaccines [21].
- However, age-specific information regarding the occurrence and characteristics of myocarditis and pericarditis in adolescents following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in Asia remains insufficient [22]. Accordingly, this study aimed to investigate the incidence and clinical characteristics of myocarditis and pericarditis in adolescents (aged 12–19 years) in South Korea following mRNA-based COVID-19 vaccination from July 19, 2021 (when COVID-19 vaccination was initiated in adolescents of the study age range) to September 30, 2022. We also analyzed the differences in clinical characteristics according to the level of severity.
Introduction
- Detection, Reporting, and Assessment of Adverse Events of Myocarditis and Pericarditis
- In Korea, physicians, medical doctors, and dentists are required to report events of diagnosed adverse reactions following vaccination through the Korea Immunization Management System (KIMS); they can also be reported by the affected vaccinated person, or by their parents or guardians [23]. The reported cases were then investigated by city or provincial government epidemiologists in order to collect additional data (e.g., clinical records, underlying diseases, lab test results, and treatment approach and outcomes), and the results were reviewed by a rapid response team [24]. Based on these results, the diagnostic certainty and causality assessment of myocarditis and pericarditis cases were finally reviewed and determined by the Adverse Event Following Immunization (AEFI) Expert Advisory Committee operated by the Korea Disease Control and Prevention Agency [24,25]. The level of diagnostic certainty was assessed using a slightly modified version of the diagnostic criteria as defined by the Brighton Collaboration case definition of myocarditis and pericarditis (Tables 1, 2) [25,26].
- Study Population
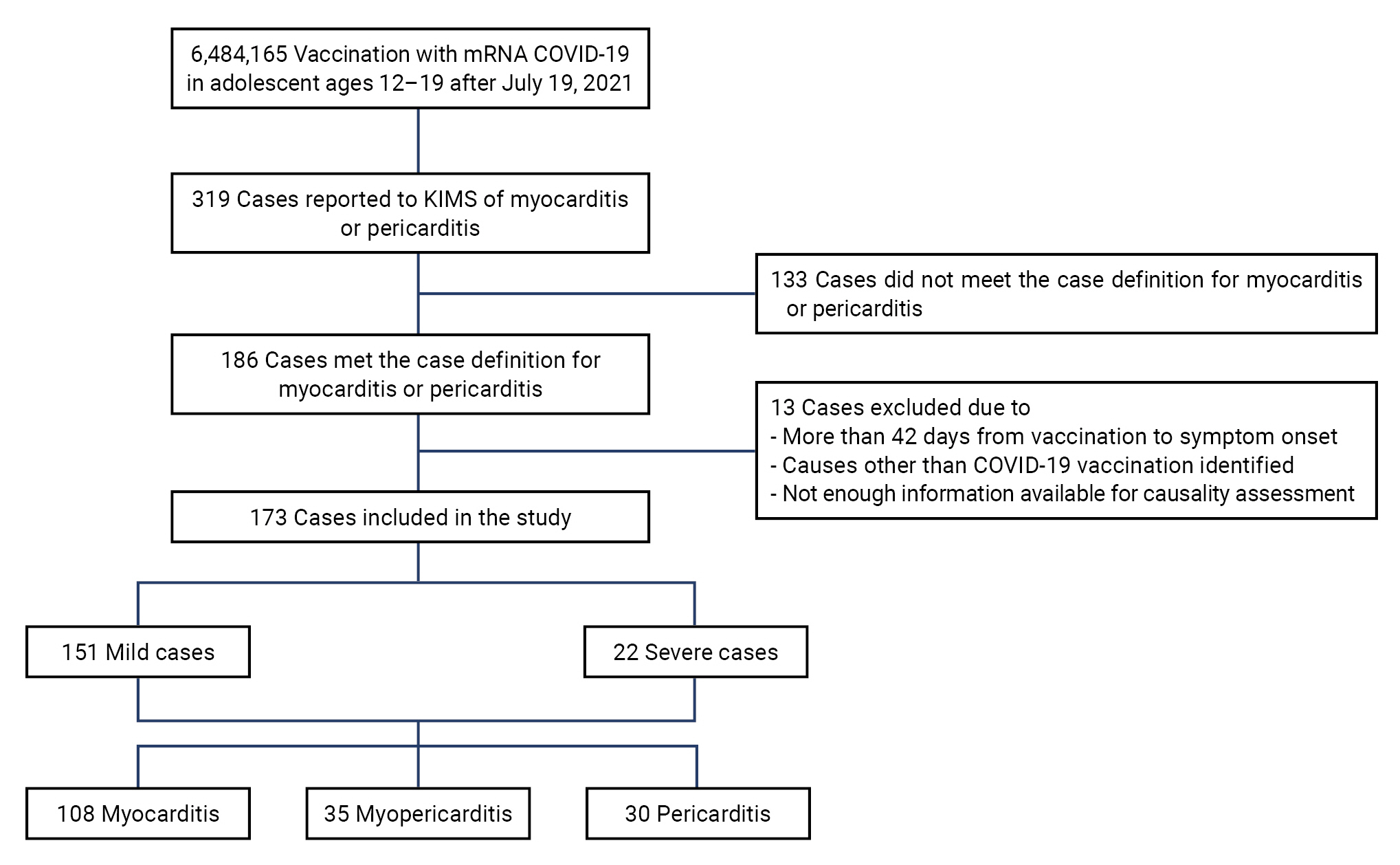
- Between July 19, 2021 and September 30, 2022, 3,728,224 individuals aged 12 to 19 years received a total of 6,484,165 mRNA-based COVID-19 vaccines, and 319 cases were reported through the KIMS as suspected myocarditis or pericarditis following vaccination. Of these 319 reported cases, 186 cases met the case definition (myocarditis: definite, possible, or probable; pericarditis: definite or probable). Of the 186 cases, we excluded 13 cases in which (1) the adverse reactions occurred more than 42 days after vaccination [27]; (2) the events were determined to have causes other than COVID-19 vaccination after review by the AEFI Expert Advisory Committee [24,25]; or (3) more information was required for causality assessment. Finally, 173 cases were selected for use in this study (Figure 1).
- Data Collection
- This retrospective descriptive study was conducted using patient data from the KIMS. We collected the following types of data: age, sex, type of vaccine, number of the vaccine, vaccination date, symptom onset dates, symptoms and signs, laboratory values (troponin I or T, creatine kinase-myocardial band [CK-MB], C-reactive protein [CRP], and erythrocyte sedimentation rate [ESR]), imaging results (electrocardiography [ECG], echocardiography, and cardiac magnetic resonance imaging [cMRI]), and other test results.
- Troponin I, troponin T, CK-MB, and CRP levels were deemed elevated if they were higher than the laboratory’s reference levels. The ESR was determined to be elevated if it exceeded 20 mm/h [28,29]. Imaging test results were classified based on the Adverse Events of Special Interest Case Definition Companion Guide of the Safety Platform for Emergency Vaccines [26]. The cases were divided into mild and severe. Severe cases were defined as death, admission to the intensive care unit (ICU), or life-threatening conditions [24]. In addition, when examining the clinical characteristics of myocarditis and pericarditis, myopericarditis cases were included in both myocarditis and pericarditis cases for analysis.
- Statistical Analysis
- For all demographic and clinical characteristics, categorical variables were presented as frequencies and percentages. The mean and standard deviation were used to present normally distributed continuous variables, while the median and interquartile range (IQR) were applied to present skewed variables. The normality of distributions was assessed with the Shapiro-Wilk test. The chi-square and Fisher exact tests were performed to compare categorical variables, and the Mann-Whitney U-test was performed to compare continuous variables. The incidence rates of myocarditis and pericarditis were estimated as the ratio between the number of outcomes and the number of person-days that occurred during the period of interest per 1,000,000 person-days, and 95% confidence intervals (CIs) were calculated. All data were analyzed using the R software ver. 4.1.2 (The R Foundation). A p-value <0.05 was considered to indicate statistical significance.
- Ethics Statement
- This study protocol was reviewed and approved by the Public Institutional Review Board (IRB) designated by the Ministry of Health and Welfare (IRB No: P01-202212-01-00). The requirement for informed consent was waived by the IRB.
Materials and Methods
- General Characteristics of 12- to 19-Year-Olds with Myocarditis and Pericarditis
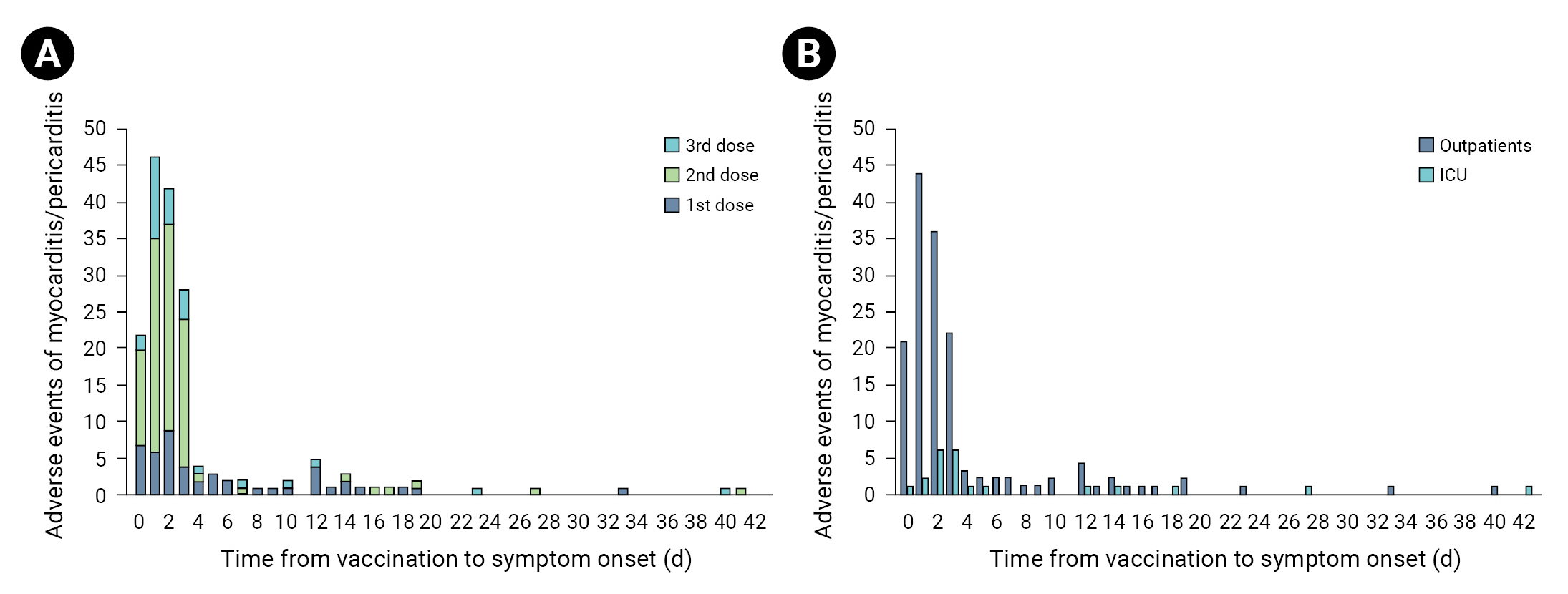
- The median age of the 173 cases was 16 years (IQR, 15–18 years). Those aged 12 to 17 years comprised 66.5% (n=115) of the cases, while those aged 18 to 19 years made up the remaining 33.5% (n=58). The frequency of the 12 to 17 year age group was about twice as high as that of the 18 to 19 year age group. In terms of sex, 924,165 adolescent males received a total of 3,342,994 vaccinations, from which 139 cases of myocarditis or pericarditis occurred; while 1,804,059 adolescent females received a total of 3,141,172 vaccinations, from which 34 cases of myocarditis or pericarditis occurred. The incidence of myocarditis or pericarditis was approximately 4 times greater in adolescent males (80.3%, n=139) than in females (19.7%, n=34). Most patients had received 2 vaccine doses (56.6%, n=98), followed by 1 dose (27.2%, n=47) and 3 doses (16.2%, n=28). Myocarditis was diagnosed in 62.4% of cases (n=108), myopericarditis in 20.2% (n=35), and pericarditis in 17.3% (n=30). Almost all of the adolescents (96.0%, n=166) received the BNT162b2 vaccine, while 4.0% (n=7) received the mRNA-1273 vaccine. The median time from vaccination to symptom onset was 2 days (IQR, 1–3 days). Most cases (87.3%, n=151) were mild, while 12.7% (n=22) were severe. No deaths were reported (Table 3).
- The incidence rate of myocarditis/pericarditis was approximately 3.8 times higher in males (0.99 per 100,000 person-days; 95% CI, 0.83–1.17 per 100,000 person-days) than in females (0.26 per 100,000 person-days; 95% CI, 0.18–0.36 per 100,000 person-days), regardless of the number of doses received. The highest rate was observed in males aged 12 to 17 years after the second dose (1.64 per 100,000 person-days; 95% CI, 1.27–2.09 per 100,000 person-days) (Table 4).
- The proportion of severe cases in females was 23.5%, which was higher, although not significantly, than that reported in males (10.1%). The proportion of severe cases was the highest in myopericarditis patients (17.1%), followed by patients diagnosed with myocarditis (14.8%). All diagnosed cases of pericarditis were mild. The median time from vaccination to symptom onset was 2 days (IQR, 1–3 days) (Table 5) in mild cases and 3 days (IQR, 2–4.75 days) in severe cases (p=0.003) (Table 5, Figure 2).
- Clinical Characteristics of 12- to 19-Year-Olds with Myocarditis and Pericarditis
- The most common clinical symptom was chest pain or pressure (93.7%, 134/143) followed by dyspnea (30.1%, 43/143) and heart palpitations (16.1%, 23/143).
- Troponin I or T was elevated in 95.8% of the tested cases (137/143), CK-MB was elevated in 67.6% (94/139), and CRP was elevated in 75.2% (100/133). Additionally, the ESR was ≥20 mm/h in 16.7% of the tested cases (14/84). The proportion of cases with elevated troponin I or T was 95.9% in mild cases (116/121) and 95.5% in severe cases (21/22), whereas the proportions of cases with elevated CK-MB (65.8% vs. 77.3%), CRP (73.9% vs. 83.3%), and ESR (10.7% vs. 66.7%, p=0.001) were higher in severe cases.
- On ECG, ST-segment or T-wave abnormalities (elevation or inversion) were found in 60.3% of cases (85/141), paroxysmal or sustained atrial or ventricular arrhythmias in 21.3% (30/141), and atrioventricular (AV) nodal conduction delays or intraventricular conduction defects in 6.4% (9/141). Paroxysmal or sustained atrial or ventricular arrhythmias were more common in severe cases than in mild cases (45.5% vs. 16.8%, p=0.008). Additionally, the proportion of cases with the finding of AV nodal conduction delays or intraventricular conduction defects was higher in severe cases than in mild cases (18.2% vs. 4.2%, p=0.034).
- On echocardiography, the proportion of patients with a left ventricular ejection fraction (LVEF) <55% was 10.6% in mild cases (12/113) and 35.3% in severe cases (6/17). Among the severe cases, 1 patient with moderate dysfunction (LVEF, 35%–44%) and 1 patient with severe dysfunction (LVEF, <35%) were identified.
- Edema on T2-weighted MRI occurred in 28.9% (13/45) of all tested cases, 21.6% of mild cases (8/37), and 62.5% of severe cases (5/8; p=0.03). Additionally, late gadolinium enhancement on T1-weighted MRI was reported in 48.9% of all tested cases (22/45), 43.2% of mild cases (16/37), and 75.0% of severe cases (6/8) (Table 6).
- Chest pain or pressure were the most common symptoms (98.5%, 64/65), followed by dyspnea (15.4%, 10/65) and heart palpitations (29.2%, 19/65). CRP was elevated in 76.3% of cases (45/59), and ESR was ≥20 mm/h in 25.0% (8/32). The proportions of elevated CRP (74.1% vs. 100.0%) and ESR (24.1% vs. 33.3%) were higher in severe cases than in mild cases, but with no statistically significant difference.
- ST-segment or T-wave abnormalities (elevation or inversion) were reported on ECG in most cases (77.8%, 49/63). Meanwhile, an ST-segment depression in augmented vector right was detected in 6.3% (4/63) of cases and PR-depression throughout the leads (best shown on leads II and V3) without reciprocal ST-segment changes (depressions) in 6.3% (4/63).
- On echocardiography findings, the proportion of patients with an LVEF <55% was 12.8% in mild cases (6/47) and 16.7% in severe cases (1/6), whereas mild dysfunction (LVEF, 45%–54%) was observed in 10.6% of mild cases (5/47) and 16.7% of severe cases (1/6), respectively.
- Imaging test results (echocardiogram, MRI, cMRI, or computed tomography) further revealed that abnormal pericardial fluid collection or pericardial inflammation occurred in 75.4% of all tested cases (49/65), 76.3% of mild cases (45/59), and 66.7% of severe cases (4/6) (Table 7).
Results
Myocarditis
Pericarditis
- In the current study, the occurrence and clinical characteristics of myocarditis and pericarditis in adolescents aged 12 to 19 years after mRNA-based COVID-19 vaccination in South Korea from July 2021 to September 2022 were examined. Myocarditis and pericarditis were identified as rare occurrences, and though most cases (87%) were mild, the incidence rate was higher in adolescent males and following the second dose.
- This finding is consistent with several previous study findings. For example, Truong et al. [30] analyzed cases of suspected myocarditis after COVID-19 vaccination in individuals under the age of 20 years from 26 pediatric medical centers in the United States and Canada. They found that 90.6% of the reported cases were in males, and 91.4% were in those who had received a second vaccine dose. These patterns were also observed before the introduction of COVID-19 vaccines. That is, Kim and Cho [31] examined the nationwide incidence, treatment, and outcomes of acute myocarditis in Korean children between 2007 and 2016 using the database of the Korea Health Insurance Review & Assessment Service (HIRA). They found that the incidence of acute myocarditis was significantly higher in boys greater than 13 years of age. Although the specific causes of these sex-based differences are unclear, high testosterone levels in boys can directly facilitate immune responses, which lead to an increased likelihood of inflammation, fibrosis, dilated cardiomyopathy, and heart failure [32].
- The median time from vaccination to symptom onset was 2 days (IQR, 1–3 days), and in most cases, symptoms occurred within 7 days. More specifically, the median time to symptom onset after the first dose was 3 days (IQR, 1–9.5 days), and that after the second dose was 2 days (IQR, 1–3 days). A similar finding was reported by Oster et al. [33], who indicated that the median time until symptom onset was 3 days (IQR, 1–8 days) and 2 days (IQR, 1–3 days) after the first and second doses, respectively. Hence, in individuals vaccinated with mRNA-based vaccines, a diagnosis of myocarditis is generally made within 2 to 3 days of vaccination [13], whereas in typical cases of viral myocarditis, symptoms often manifest within a few weeks to a few months [34].
- The current study also found that 12.7% of the cases were considered severe, with no deaths reported. Meanwhile, Kim and Cho [31] conducted a study on the nationwide incidence, treatment, and outcomes of acute myocarditis in Korean children based on the 2007–2016 HIRA database. They reported that 22.8% of acute myocarditis pediatric patients required extracorporeal membrane oxygenation, or ventilator support, and 6.9% died.
- With respect to clinical symptoms, most cases manifested as chest pain or pressure, which agrees with the findings of previous studies [33]. Of note, severe cases reported manifestations of nonspecific symptoms, such as stomachache or vomiting, without specific symptoms, such as chest pain or pressure. Considering that nonspecific symptoms can be mistaken for gastrointestinal (GI) issues (e.g., viral gastroenteritis), misdiagnoses can occur if based solely on symptoms without suspecting myocarditis [35]. Furthermore, several studies have indicated that cases of myocarditis accompanied by GI symptoms in children were closely related to ICU admission and fatal outcomes [20,36,37], so it is clinically important to screen children presenting with GI symptoms for myocarditis [37]. Reportedly, myocarditis accompanied by GI symptoms may be caused by poor perfusion to the digestive system due to cardiac dysfunction, or by infection of the GI tract by the same virus causing myocarditis [38].
- Troponin I or T was elevated in 96% of the tested cases. Similarly, elevated troponin I or T levels were previously reported in 98% of patients under the age of 30 years by Oster et al. [33], and in 100% of patients by Truong et al. [30]. Elevation of troponin I, which is expressed in cardiac muscles, has high sensitivity and high specificity for detecting damage to the myocardium [39,40]. CRP levels were elevated in 75.2% and 76.3% of the cases diagnosed with myocarditis and pericarditis, respectively, while the ESR was ≥20 mm/h in 16.7% and 25.0% of the cases. A similar degree of incongruence between CRP and ESR trends was reported by Marshall et al. [14], who reported 7 cases of acute myocarditis and myopericarditis in male adolescents who complained of chest pain within 4 days of vaccination with BNT162b2-BioNTech COVID-19 vaccines. In their study, CRP levels were elevated in 6/7 cases, while the mean ESR was 18.29±14.95 mm/h, suggesting that CRP is a more sensitive marker of inflammation than ESR [41,42]. While CRP begins to increase 4–6 hours after inflammation begins, and peaks 2 to 3 days later, ESR levels tend to rise and fall more slowly, thus causing a disparity in the levels of these 2 factors [43].
- In patients with myocarditis, various abnormalities are detected via ECG, including ST-segment or T-wave, Q waves, AV block, and bundle branch blocks. In agreement with previous studies [21,22], the most common finding in this study was ST-segment or T-wave abnormalities (elevation or inversion). Similarly, Witberg et al. [44] reported abnormal ECG findings in 67% of adolescents diagnosed with myocarditis related to vaccination with BNT162b2. Additionally, Jain et al. [18] assessed patients aged ≤21 years and diagnosed with myocarditis related to COVID-19 vaccination across 16 hospitals in the US. They found that 70% of the patients exhibited abnormalities in ECG results, with ST-segment or T-wave abnormalities (elevation or inversion) determined to be the most common. In the current study, the occurrence of paroxysmal or sustained atrial or ventricular arrhythmias was more common in severe than in mild, cases (45.5% vs. 16.8%, p=0.008). This was also found in a previous study that reported that 25% of acute myocarditis cases exhibited cardiac arrhythmia, which was more frequently observed in severe myocarditis [45,46]. Furthermore, in the current study, AV nodal conduction delays or intraventricular conduction defects were more frequently observed in severe cases compared with mild cases (18.2% vs. 4.2%, p=0.034). Indeed, high-grade AV block is associated with higher morbidity and mortality rates in myocarditis patients [47].
- On echocardiography, an LVEF <55% was observed in 13.9% and 13.3% of myocarditis and pericarditis cases, respectively. Meanwhile, in a meta-analysis of 24 studies on myocarditis related to BNT162b2 and mRNA‐1273 COVID‐19 vaccines, Woo et al. [20] reported that the median LVEF in myocarditis patients aged ≤20 years was 56.8% (43.7%–64.7%). Truong et al. [30] further reported that 62 cases (82.7%) with cMRI abnormalities had normal LVEF. In contrast, in the current study, of the cases with severe myocarditis, 1 case each was found to have moderate dysfunction (LVEF, 35%–44%) and severe dysfunction (LVEF, <35%). Importantly, a retrospective study conducted among 320 patients diagnosed with acute myocarditis reported a mean LVEF of 54%±9%, and found that, in comparison to patients with normal LVEF, those with decreased LVEF were more likely to receive steroid therapy during hospital stays and experience cardiovascular complications [48].
- cMRI is a noninvasive diagnostic method that is highly effective in the diagnosis of myocarditis [49]. Schauer et al. [50] analyzed 16 patients aged 12 to 16 years who had been diagnosed with myopericarditis after receiving the BNT162b2 COVID-19 mRNA vaccine and received MRI within a median time of 2 days after symptom onset. All 16 patients presented with evidence of edema on T2-weighted imaging, and 93.8% (15/16) had late gadolinium enhancement in a patchy subpericardial to transmural pattern with a predilection for the inferior left ventricular free wall. However, no patient had pericardial effusion. Meanwhile, Truong et al. [30] reported that 55.7% of the patients for whom cMRI was conducted showed myocardial edema, and 76.3% had late gadolinium enhancement (median time from symptom onset to test, 5 days; IQR, 3–17 days). Meanwhile, in the current study, myocardial edema on T2-weighted MRI was observed in 28.9% of the cases, late gadolinium enhancement on T1-weighted MRI was detected in 48.9%, and 26.7% did not exhibit any abnormalities on their MRI scans. According to previous studies, in patients with acute myocarditis, cMRI markers of myocardial inflammation demonstrated a rapid and continuous decrease. Therefore, if myocarditis is suspected, cMRI scanning should be performed at an early stage of the disease [51], within 14 days [52].
- Some studies have indicated that myocarditis caused by COVID-19 infection is more common than that induced following vaccination [53–55]. For instance, Fronza et al. [55] differentiated myocarditis occurring after COVID-19 vaccination from that occurring after COVID-19 or other viral infections and compared them with other causes of myocarditis. The pattern of cardiac damage observed on the MRI scans of patients diagnosed with myocarditis after COVID-19 vaccination was similar to that of patients with myocarditis due to other causes; however, the myocardial abnormalities were less severe in patients with vaccine-related myocarditis (e.g., less functional impairment, lower native T1, and less frequent involvement of the septum). Moreover, according to a recent study performed by the United States Center for Disease Control and Prevention, in adolescents aged 12 to 17 years (i.e., an age group considered to have high cardiac risk), myocarditis or pericarditis occurred in 50 people per 100,000 after COVID-19 infection, and in 22 people per 100,000 after the second vaccine dose. It was further reported that mRNA-based COVID-19 vaccines were associated with a risk for adverse reactions, including myocarditis; however, the absolute risk level was low, and the adverse reactions were largely mild, with patients recovering rapidly.
- Our study has several limitations. First, the quantity and quality of information, which was collected from medical charts or interviews, may have varied depending on the collected data and the healthcare environment in the region. There may also have been unobserved clinical findings. Considering these points, overgeneralization should be avoided when interpreting the results of this study. Second, the criteria for defining myocarditis or pericarditis after vaccination in each country may be slightly different, but this study did not consider these details in the comparison.
Discussion
- This study investigated the incidence and clinical characteristics of myocarditis and pericarditis in adolescents aged 12 to 19 years in South Korea following mRNA-based COVID-19 vaccination using patient data from the KIMS from July 2021 to September 2022. Myocarditis and pericarditis have been reported as rare occurrences following mRNA-based COVID-19 vaccination. Most cases were mild, but the incidence rate was particularly higher in adolescent males and after the second dose. As bivalent severe acute respiratory syndrome coronavirus 2 mRNA vaccination against the Omicron variants started in South Korea in October 2022, the development of myocarditis and pericarditis after vaccination should be monitored closely considering clinical characteristics [23]. Further comprehensive research, including studies of the incidence and pathophysiology of myocarditis and pericarditis in Korean adolescents after vaccination with COVID-19, as well as the treatment and prognosis of these conditions, should be conducted in the future.
Conclusion
- • The age-specific information regarding myocarditis and pericarditis in adolescents following mRNA-based COVID-19 vaccination in Asia remains insufficient. This study aimed to investigate the incidence and clinical characteristics of myocarditis and pericarditis in adolescents in South Korea following mRNA-based COVID-19 vaccination.
- • Following mRNA-based COVID-19 vaccination, myocarditis and pericarditis has been reported as a rare, most cases were mild, but the incidence rate was particularly higher in men, and after the second dose.
- • As bivalent SARS-CoV-2 mRNA vaccination started in South Korea from October 2022, development of myocarditis and pericarditis after vaccination should be monitored closely considering clinical characteristic.
HIGHLIGHTS
-
Ethics Approval
This study was reviewed and approved by the Public Institutional Review Board designated by the Ministry of Health and Welfare (IRB No: P01-202212-01-00) and performed in accordance with the principles of the Declaration of Helsinki.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: all authors; Data curation: JYS, SYK; Formal analysis JYS, SYK; Methodology: all authors; Project administration: JYS; Supervision: EKK, SYK; Visualization: JYS; Writing–original draft: JYS; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Article information
Based on Korea Disease Control and Prevention Agency [25].
These criteria for diagnostic comparability were used for the purposes of early assessment and case collection. Final decisions regarding diagnostic compatibility and causality followed the decisions of experts and the vaccine adverse event evaluation team.
cMRI, cardiac magnetic resonance imaging; CK-MB, creatine kinase-myocardial band; ECG, electrocardiography.
Based on Korea Disease Control and Prevention Agency [25].
These criteria for diagnostic comparability were used for the purposes of early assessment and case collection. Final decisions regarding diagnostic compatibility and causality followed the decisions of experts and the vaccine adverse event evaluation team.
ECG, electrocardiography.
| Characteristic | Total | Dose 1 | Dose 2 | Dose 3 |
|---|---|---|---|---|
| No. of vaccination doses administered | 6,484,165 | 2,826,964 | 2,746,110 | 910,282 |
| No. of cases that met the case definition for myocarditis or pericarditis | 173 (100.0) | 47 (27.2) | 98 (56.6) | 28 (16.2) |
| Age group (y) | ||||
| 12–17 | 115 (66.5) | 33 (28.7) | 74 (64.3) | 8 (7.0) |
| 18–19 | 58 (33.5) | 14 (24.1) | 24 (41.4) | 20 (34.5) |
| Sex | ||||
| Male | 139 (80.3) | 34 (24.5) | 82 (59.0) | 23 (16.5) |
| Female | 34 (19.7) | 13 (38.2) | 16 (47.1) | 5 (14.7) |
| Adjudication diagnosis | ||||
| Myocarditis | 108 (62.4) | 30 (27.8) | 65 (60.2) | 13 (12.0) |
| Myopericarditis | 35 (20.2) | 9 (25.7) | 17 (48.6) | 9 (25.7) |
| Pericarditis | 30 (17.3) | 8 (26.7) | 16 (53.3) | 6 (20.0) |
| Type of vaccine | ||||
| BNT162b2 | 166 (96.0) | 45 (27.1) | 94 (56.6) | 27 (16.3) |
| mRNA-1273 | 7 (4.0) | 2 (28.6) | 4 (57.1) | 1 (14.3) |
| Time from vaccination to symptom onset (d) | 2 (1–3) | 3 (1–9.5) | 2 (1–3) | 2 (1–3) |
| Severity | ||||
| Mild case | 151 (87.3) | 40 (26.5) | 85 (56.3) | 26 (17.2) |
| Severe casea) | 22 (12.7) | 7 (31.8) | 13 (59.1) | 2 (9.1) |
| Death | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Characteristic | Total (n=173) | Mild case (n=151) | Severe case (n=22)a) | p-value |
|---|---|---|---|---|
| Age group (y) | ||||
| 12–17 | 115 (66.5) | 98 (85.2) | 17 (14.8) | 0.36 |
| 18–19 | 58 (33.5) | 53 (91.4) | 5 (8.6) | |
| Sex | ||||
| Male | 139 (79.8) | 125 (89.9) | 14 (10.1) | 0.06 |
| Female | 34 (19.7) | 26 (76.5) | 8 (23.5) | |
| Adjudication of the diagnosis | ||||
| Myocarditis | 108 (62.4) | 92 (85.2) | 16 (14.8) | 0.07 |
| Myopericarditis | 35 (20.2) | 29 (82.9) | 6 (17.1) | |
| Pericarditis | 30 (17.3) | 30 (100.0) | 0 (0) | |
| Type of vaccine | ||||
| BNT162b2 | 166 (96.0) | 144 (86.7) | 22 (13.3) | 0.59 |
| mRNA-1273 | 7 (4.0) | 7 (100.0) | 0 (0) | |
| Dose | ||||
| 1 | 47 (27.2) | 40 (85.1) | 7 (14.9) | 0.58 |
| 2 | 98 (56.6) | 85 (86.7) | 13 (13.3) | |
| 3 | 28 (16.2) | 26 (92.9) | 2 (7.1) | |
| Time from vaccination to symptom onset (d) | 2 (1–3) | 2 (1–3) | 3 (2–4.75) | 0.003** |
| Characteristic | Total (n=143) | Mild case (n=121) | Severe case (n=22)a) | p-value |
|---|---|---|---|---|
| Symptoms (n=143) | ||||
| Acute chest pain or pressure | 134/143 (93.7) | 114/121 (94.2) | 20/22 (90.9) | 0.91 |
| Dyspnea after exercise, at rest, or lying down | 43/143 (30.1) | 35/121 (28.9) | 8/22 (36.4) | 0.65 |
| Palpitation | 23/143 (16.1) | 20/121 (16.5) | 3/22 (13.6) | <0.999 |
| Diaphoresis | 2/143 (1.4) | 2/121 (1.7) | 0/22 (0) | <0.999 |
| Nonspecific symptom (fever, mental change, abdominal pain, nausea, vomiting) | 2/143 (1.4) | 0/121 (0) | 2/22 (9.1) | <0.999 |
| Laboratory values | ||||
| Myocardial biomarker | ||||
| Elevated troponin I or T (n=143) | 137/143 (95.8) | 116/121 (95.9) | 21/22 (95.5) | <0.999 |
| Elevated CK-MB (n=139) | 94/139 (67.6) | 77/117 (65.8) | 17/22 (77.3) | 0.42 |
| Inflammation biomarker | ||||
| Elevated CRP (n=133) | 100/133 (75.2) | 85/115 (73.9) | 15/18 (83.3) | 0.56 |
| Elevated ESR (n=84) | 14/84 (16.7) | 8/75 (10.7) | 6/9 (66.7) | <0.001*** |
| Testing/imaging | ||||
| ECG (n=141) | ||||
| ST-segment or T-wave abnormalities (elevation or inversion) | 85/141 (60.3) | 70/119 (58.8) | 15/22 (68.2) | 0.56 |
| Paroxysmal or sustained atrial or ventricular arrhythmias | 30/141 (21.3) | 20/119 (16.8) | 10/22 (45.5) | 0.008* |
| AV nodal conduction delays or intraventricular conduction defects | 9/141 (6.4) | 5/119 (4.2) | 4/22 (18.2) | 0.034* |
| Continuous ambulatory electrocardiographic monitoring that detects frequent atrial or ventricular ectopy | 0/141 (0) | 0/119 (0) | 0/22 (0) | - |
| Echocardiogram, LVEF (n=130) | ||||
| Normal (≥55%) | 112/130 (86.1) | 101/113 (89.4) | 11/17 (64.7) | 0.004** |
| Mild dysfunction (45%–54%) | 16/130 (12.3) | 12/113 (10.6) | 4/17 (23.5) | |
| Moderate dysfunction (35%–44%) | 1/130 (0.8) | 0/113 (0) | 1/17 (5.9) | |
| Severe dysfunction (<35%) | 1/130 (0.8) | 0/113 (0) | 1/17 (5.9) | |
| Cardiac MRI (n=45) | ||||
| Edema on T2-weighted study, typically patchy in nature | 13/45 (28.9) | 8/37 (21.6) | 5/8 (62.5) | 0.03 |
| Late gadolinium enhancement on T1-weighted study with an increased enhancement ratio between myocardial and skeletal muscle, typically involving at least one non-ischemic regional distribution with recovery (myocyte injury) | 22/45 (48.9) | 16/37 (43.2) | 6/8 (75.0) | 0.13 |
Data are presented as n (%). Abnormality was defined per the reference range of the hospital or laboratory where the test was performed.
ECG, electrocardiography; CK-MB, creatine kinase-myocardial band; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; AV, atrioventricular; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging.
a) Intensive care unit admission or life-threatening condition.
* p<0.05,
** p<0.01,
*** p<0.001.
| Characteristic | Total (n=65) | Mild case (n=59) | Severe case (n=6)a) | p-value |
|---|---|---|---|---|
| Symptoms (n=65) | ||||
| Acute chest pain or pressure | 64/65 (98.5) | 58/59 (98.3) | 6/6 (100.0) | <0.999 |
| Dyspnea after exercise, at rest, or lying down | 19/65 (29.2) | 18/59 (30.5) | 1/6 (16.7) | 0.66 |
| Palpitation | 10/65 (15.4) | 9/59 (15.3) | 1/6 (16.7) | <0.999 |
| Diaphoresis | 0/65 (0) | 0/59 (0) | 0/6 (0) | - |
| Laboratory values | ||||
| Inflammation biomarker | ||||
| Elevated CRP (n=59) | 45/59 (76.3) | 40/54 (74.1) | 5/5 (100.0) | 0.33 |
| Elevated ESR (n=32) | 8/32 (25.0) | 7/29 (24.1) | 1/3 (33.3) | <0.999 |
| Testing/imaging | ||||
| ECG (n=63) | ||||
| ST-segment or T-wave abnormalities (elevation or inversion) | 49/63 (77.8) | 43/57 (75.4) | 6/6 (100.0) | 0.32 |
| ST-segment depression in aVR | 4/63 (6.3) | 3/57 (5.3) | 1/6 (16.7) | 0.34 |
| PR-depression throughout the leads (best shown in leads II & V3) without reciprocal ST-segment changes (depressions) | 4/63 (6.3) | 3/57 (5.3) | 1/6 (16.7) | 0.34 |
| Echocardiogram, LVEF (n=53) | ||||
| Normal (≥55%) | 46/53 (86.7) | 41/47 (87.2) | 5/6 (83.3) | 0.54 |
| Mild dysfunction (45%–54%) | 6/53 (11.3) | 5/47 (10.6) | 1/6 (16.7) | |
| Moderate dysfunction (35%–44%) | 0/53 (0) | 0/47 (0) | 0/6 (0) | |
| Severe dysfunction (<35%) | 0/53 (0) | 0/47 (0) | 0/6 (0) | |
| Cardiac imaging (echocardiogram, MRI, cardiac MRI, CT) (n=65) | ||||
| Abnormal pericardial fluid collection or pericardial inflammation | 49/65 (75.4) | 45/59 (76.3) | 4/6 (66.7) | 0.62 |
Data are presented as n (%). Abnormality was defined per the reference range of the hospital or laboratory where the test was performed.
ECG, electrocardiography; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; aVR, augmented vector right; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; CT, computed tomography.
a) Intensive care unit admission or life-threatening condition.
- 1. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347−58.ArticlePubMedPMC
- 2. Most ZM, Hendren N, Drazner MH, et al. Striking similarities of multisystem inflammatory syndrome in children and a myocarditis-like syndrome in adults: overlapping manifestations of COVID-19. Circulation 2021;143:4−6.ArticlePubMed
- 3. Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 2020;78:185−93.ArticlePubMedPMC
- 4. Cauchemez S, Bosetti P, Kiem CT, et al. Education and mental health: good reasons to vaccinate children. Lancet 2021;398:387. ArticlePubMedPMC
- 5. Korea Disease Control and Prevention Agency (KDCA). Press releases: from July to the end of September, at least one vaccination is given to citizens aged 18 years or older (June 18, 2021, regular briefing) [Internet]. KDCA; 2021 [cited 2022 Dec 2]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=713689&cg_code=C01&act=view&nPage=1. Korean.
- 6. Korea Disease Control and Prevention Agency (KDCA). Press releases: adolescents aged 12-17 years vaccination (August 30, 2021, regular briefing) [Internet]. KDCA; 2021 [cited 2022 Dec 2]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=716707&cg_code=C0&act=view&nPage=11. Korean.
- 7. Korea Disease Control and Prevention Agency (KDCA). Press releases: adolescents aged 16-17 years vaccination (October 18, 2021, regular briefing) [Internet]. KDCA; 2021 [cited 2022 Dec 2]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=717277&cg_code=C01&act=view&nPage=1. Korean.
- 8. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384:2187−201.ArticlePubMedPMC
- 9. Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med 2022;386:340−50.ArticlePubMedPMC
- 10. Mohammed I, Nauman A, Paul P, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother 2022;18:2027160. ArticlePubMedPMC
- 11. Kuehn BM. Adolescent myocarditis after COVID-19 vaccination is rare. JAMA 2021;326:902. Article
- 12. Li M, Wang X, Feng J, et al. Myocarditis or pericarditis following the COVID-19 vaccination in adolescents: a systematic review. Vaccines (Basel) 2022;10:1316. ArticlePubMedPMC
- 13. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471−84.ArticlePubMedPMC
- 14. Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics 2021;148:e2021052478.ArticlePubMedPDF
- 15. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices-United States, June 2021. MMWR Morb Mortal Wkly Rep 2021;70:977−82.ArticlePubMedPMC
- 16. Ilonze OJ, Guglin ME. Myocarditis following COVID-19 vaccination in adolescents and adults: a cumulative experience of 2021. Heart Fail Rev 2022;27:2033−43.ArticlePubMedPMCPDF
- 17. Hause AM, Gee J, Baggs J, et al. COVID-19 vaccine safety in adolescents aged 12-17 years: United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1053−8.ArticlePubMedPMC
- 18. Jain SS, Steele JM, Fonseca B, et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics 2021;148:e2021053427.ArticlePubMedPDF
- 19. Li X, Lai FT, Chua GT, et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatr 2022;176:612−4.ArticlePubMedPMC
- 20. Woo W, Kim AY, Yon DK, et al. Clinical characteristics and prognostic factors of myocarditis associated with the mRNA COVID-19 vaccine. J Med Virol 2022;94:1566−80.ArticlePubMedPMCPDF
- 21. Park H, Yun KW, Kim KR, et al. Epidemiology and clinical features of myocarditis/pericarditis before the introduction of mRNA COVID-19 vaccine in Korean children: a multicenter study. J Korean Med Sci 2021;36:e232.ArticlePubMedPMCPDF
- 22. Chua GT, Kwan MY, Chui CS, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clin Infect Dis 2022;75:673−81.ArticlePubMedPMCPDF
- 23. Ministry of Health and Welfare (MOHW). Press releases: from December 12, additional vaccination for the winter season for teenagers aged 12-17 begins [Internet]. MOHW; 2022 [cited 2022 Dec 25]. Available from: https://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&page=1&CONT_SEQ=374022. Korean.
- 24. Korea Disease Control and Prevention Agency (KDCA). Adverse event management guidelines following COVID-19 vaccination. 2-2th ed. [Internet]. KDCA; 2022 [cited 2022 Dec 12]. Available from: https://ncv.kdca.go.kr/upload_comm/syview/doc.html?fn=165810864658800.pdf&rs=/upload_comm/docu/0031/. Korean.
- 25. Korea Disease Control and Prevention Agency (KDCA). Myocarditis and pericarditis after COVID-19 vaccination guidelines: for health care personnel. 2-1th ed. [Internet]. KDCA; 2021 [cited 2022 Dec 12]. Available from: https://ncv.kdca.go.kr/board.es?mid=a12101000000&bid=0031&act=view&list_no=638&tag=&nPage=1.
- 26. Law B. SO2-D2. 5.2. 2-AESI case definition companion guide for 2nd tier AESI [Internet]. Safety Platform for Emergency vACcines (SPEAC); 2022 [cited 2023 Mar 20]. Available from: https://brightoncollaboration.us/wp-content/uploads/2022/05/SPEAC_D2.5.2.2_Myocarditis-companion-guide_codes-updated_BL_2022_May12.pdf.
- 27. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078−90.ArticlePubMedPMC
- 28. Long SS, Pickering LK, Prober CG. Principles and practice of pediatric infectious diseases. 4th ed. Elsevier; 2012.
- 29. Ramsay ES, Lerman MA. How to use the erythrocyte sedimentation rate in paediatrics. Arch Dis Child Educ Pract Ed 2015;100:30−6.ArticlePubMed
- 30. Truong DT, Dionne A, Muniz JC, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation 2022;145:345−56.ArticlePubMed
- 31. Kim J, Cho MJ. Acute myocarditis in children: a 10-year nationwide study (2007-2016) based on the Health Insurance Review and Assessment Service Database in Korea. Korean Circ J 2020;50:1013−22.ArticlePubMedPMCPDF
- 32. Giron-Gonzalez JA, Moral FJ, Elvira J, et al. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol 2000;143:31−6.ArticlePubMed
- 33. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-Based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022;327:331−40.PubMedPMC
- 34. Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006;114:1581−90.ArticlePubMed
- 35. Freedman SB, Haladyn JK, Floh A, et al. Pediatric myocarditis: emergency department clinical findings and diagnostic evaluation. Pediatrics 2007;120:1278−85.ArticlePubMedPDF
- 36. Hsiao HJ, Hsia SH, Wu CT, et al. Clinical presentation of pediatric myocarditis in Taiwan. Pediatr Neonatol 2011;52:135−9.ArticlePubMed
- 37. Chang YJ, Hsiao HJ, Hsia SH, et al. Analysis of clinical parameters and echocardiography as predictors of fatal pediatric myocarditis. PLoS One 2019;14:e0214087.ArticlePubMedPMC
- 38. Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet 2000;356:930−3.ArticlePubMed
- 39. Smith SC, Ladenson JH, Mason JW, et al. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997;95:163−8.ArticlePubMed
- 40. Lauer B, Niederau C, Kuhl U, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol 1997;30:1354−9.ArticlePubMed
- 41. Fransen J, Welsing PM, De Keijzer RM, et al. Disease activity scores using C-reactive protein: CRP may replace ESR in the assessment of RA disease activity. Ann Rheum Dis 2004;62 Suppl 1:151.
- 42. Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation rate and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum 2012;41:866−71.ArticlePubMedPMC
- 43. Hilliard NJ, Waites KB. CRP ESR: C-reactive protein and ESR: what can one test tell you that the other can't? Contemp Pediatr 2002;19:64.
- 44. Witberg G, Magen O, Hoss S, et al. Myocarditis after BNT162b2 vaccination in Israeli adolescents. N Engl J Med 2022;387:1816−7.ArticlePubMedPMC
- 45. Rosier L, Zouaghi A, Barre V, et al. High risk of sustained ventricular arrhythmia recurrence after acute myocarditis. J Clin Med 2020;9:848. ArticlePubMedPMC
- 46. Sozzi FB, Gherbesi E, Faggiano A, et al. Viral myocarditis: classification, diagnosis, and clinical implications. Front Cardiovasc Med 2022;9:908663. ArticlePubMedPMC
- 47. Caughey RW, Humphrey JM, Thomas PE. High-degree atrioventricular block in a child with acute myocarditis. Ochsner J 2014;14:244−7.PubMedPMC
- 48. Younis A, Matetzky S, Mulla W, et al. Epidemiology characteristics and outcome of patients with clinically diagnosed acute myocarditis. Am J Med 2020;133:492−9.ArticlePubMed
- 49. Sanchez Tijmes F, Thavendiranathan P, Udell JA, et al. Cardiac MRI assessment of nonischemic myocardial inflammation: state of the art review and update on myocarditis associated with COVID-19 vaccination. Radiol Cardiothorac Imaging 2021;3:e210252.PubMedPMC
- 50. Schauer J, Buddhe S, Gulhane A, et al. Persistent cardiac magnetic resonance imaging findings in a cohort of adolescents with post-coronavirus disease 2019 mRNA vaccine myopericarditis. J Pediatr 2022;245:233−7.ArticlePubMedPMC
- 51. Luetkens JA, Homsi R, Dabir D, et al. Comprehensive cardiac magnetic resonance for short-term follow-up in acute myocarditis. J Am Heart Assoc 2016;5:e003603.ArticlePubMedPMC
- 52. Monney PA, Sekhri N, Burchell T, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart 2011;97:1312−8.ArticlePubMed
- 53. Voleti N, Reddy SP, Ssentongo P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: a systematic review and meta-analysis. Front Cardiovasc Med 2022;9:951314. ArticlePubMedPMC
- 54. Patone M, Mei XW, Handunnetthi L, et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation 2022;146:743−54.ArticlePubMedPMC
- 55. Fronza M, Thavendiranathan P, Chan V, et al. Myocardial injury pattern at MRI in COVID-19 vaccine-associated myocarditis. Radiology 2022;304:553−62.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Responses to Common Misconceptions Relating to COVID-19 Variant-Adapted mRNA Vaccines
George Kassianos, Pauline MacDonald, Ivan Aloysius, Shanti Pather
Vaccines.2024; 12(1): 57. CrossRef - To become a more stronger and safer country
Jong-Koo Lee
Osong Public Health and Research Perspectives.2023; 14(2): 67. CrossRef


 Cite
Cite