Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 12(5); 2021 > Article
-
Original Article
Yes-associated protein 1 as a prognostic biomarker and its correlation with telomerase in various cancers -
Hye-Ran Kim1
 , Choong-Won Seo1
, Choong-Won Seo1 , Keunje Yoo2
, Keunje Yoo2 , Sang Jun Han3
, Sang Jun Han3 , Jongwan Kim1
, Jongwan Kim1
-
Osong Public Health and Research Perspectives 2021;12(5):324-332.
DOI: https://doi.org/10.24171/j.phrp.2021.0207
Published online: September 17, 2021
1Department of Biomedical Laboratory Science, Dong-Eui Institute of Technology, Busan, Korea
2Department of Environmental Engineering, Korea Maritime and Ocean University, Busan, Korea
3Department of Biotechnology, College of Fisheries Sciences, Pukyong National University, Busan, Korea
- Corresponding author: Jongwan Kim Department of Biomedical Laboratory Science, Dong-Eui Institute of Technology, 54 Yangji-ro, Busanjin-gu, Busan, 47230, Korea E-mail: dahyun@dit.ac.kr
© 2021 Korea Disease Control and Prevention Agency
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 4,938 Views
- 94 Download
Abstract
-
Objectives
- The aims of this study were to investigate the expression of Yes-associated protein 1 (YAP1), its prognostic significance, and the correlation between YAP1 and telomerase in various cancers.
-
Methods
- The Gene Expression Profiling Interactive Analysis database was used to analyze RNA sequencing data and the survival rate of patients with various cancers in The Cancer Genome Atlas (TCGA) database. PrognoScan was used to analyze the prognostic value of YAP1 expression in various cancers. Tumor Immune Estimation Resource was used to determine the correlation between YAP1 expression and telomerase in various cancer types based on TCGA data.
-
Results
- The analysis suggested that YAP1 was differentially expressed between tissues of various cancers and non-tumor tissues. High YAP1 expression was also related to a poor prognosis in adrenocortical carcinoma, bladder urothelial carcinoma, and pancreatic adenocarcinoma. Moreover, YAP1 expression was correlated with the expression of telomerase reverse transcriptase and telomerase RNA component in various cancer types.
-
Conclusion
- These results suggest that YAP1 is a potential biomarker with prognostic significance and relevance for oncogene research in various cancer types. The correlation between the expression of YAP1 and telomere-associated genes will help to understand their cancer-promoting mechanisms and interactions.
- Yes-associated protein 1 (YAP1) expression and nuclear localization have been found to be increased in various cancers [1−4]. YAP1 is one of the most important effectors of the Hippo signaling pathway and is involved in crosstalk with other cancer-promoting pathways. The Hippo pathway plays a crucial role in organ size control and tissue regeneration [5]. The roles of Hippo pathway dysregulation in tumorigenesis and cancer progression have been widely reported [6]. As a potent oncogene activated in many cancers, YAP1 has a negatively regulated downstream target in the Hippo signaling pathway and functions as a transcriptional coactivator involved in the regulation of cell growth, proliferation, and apoptosis [2,7−9]. YAP1 plays a key role as a tumor suppressor in the Hippo signaling pathway and enhances gene transcription by binding to transcription factors [10]. Specifically, YAP1 contributes to cancer development by promoting malignant phenotypes, the expansion of cancer stem cells, and drug resistance of cancer cells. YAP1 is considered a potent oncogene closely linked to the progression of several cancer types [11,12], and YAP1 overexpression in cancer cell lines can also promote tumor growth [13,14]. Therefore, YAP1 promotes tumorigenesis, but the underlying mechanisms by which YAP1 exerts this effect require further exploration.
- Telomeres are cellular nucleoprotein complexes, and their main function is to maintain chromosomal integrity and genomic stability [15]. A telomere is a ribonucleoprotein complex composed of 2 main core subunits: telomerase reverse transcriptase (TERT), which constitutes the catalytic subunit, and a functional telomerase RNA component (TR or TERC) that provides a template for telomerase elongation [16]. A positive correlation between TERT mRNA levels and telomerase activity has been reported, suggesting that telomerase is primarily regulated by TERT expression [17]. Telomerase is active in adult germ-line tissues, immortal cells [18], and most malignant tumors [19]. TERT induces stemness of cancer cells to promote metastasis and recurrence [20]. In cancer cells, the upregulation of TERT transcriptional activity has been reported [21]. TERT overexpression has been detected in more than 80% to 90% of human cancers [15]. Thus, TERT overexpression may represent the mechanism by which cancer cells prevent telomere shortening and become immortal [22]. Zhang et al. [23] recently reported that YAP1 regulates TERT expression, and that hyperactivation of YAP1 promotes telomerase activity and increases telomeric length, causing an increase in TERT expression. They also showed that TERT expression was positively correlated with YAP1 activation in liver cancer tissues. Several studies have reported that TERT overexpression contributes to cancer progression [24]. Therefore, YAP1 promotes TERT expression, which may contribute to tumor progression [25]. However, several studies have highlighted the importance of TERC in cancer because of findings indicating that TERC expression may be highly upregulated in a variety of cancers [26−30]. Although TERC is associated with the development of several diseases, its underlying mechanisms in cancer are poorly understood. In addition, the correlation between the expression of YAP1 and telomerase-associated genes in cancer has not been completely explored.
- In the present study, we analyzed YAP1 expression in normal and different types of tumor tissues based on The Cancer Genome Atlas (TCGA) data using online databases and tools. We also evaluated the prognostic value of YAP1 expression its correlation with the expression of 2 major telomerase components (TERT and TERC) in various cancer types on the basis of TCGA data.
Introduction
- Gene Expression Profiling Interactive Analysis Database Analysis
- The Gene Expression Profiling Interactive Analysis (GEPIA) database (https://gepia.cancer-pku.cn/index.html), which is a web server tool consisting of 8,587 normal and 9,736 tumor tissue samples from the TCGA and GTEx projects [31−33], was used to analyze differences in YAP1 expression between normal and tumor tissue based on RNA sequencing. We represented expression of the YAP1 profile across various cancers and paired normal tissues. We also analyzed the survival curves, including overall survival (OS), which refers to the duration of patient survival from the date of disease treatment, and disease-free survival (DFS), which denotes relapse-free survival, according to YAP1 gene expression by using the log-rank and Mantel-Cox tests for different cancer types via the GEPIA database.
- PrognoScan Database Analysis
- The PrognoScan database (http://www.abren.net/PrognoScan/), a platform for evaluating potential tumor markers, is widely used to evaluate biological relationships between gene expression and patient prognosis such as OS and DFS [34]. It includes a large-scale collection of publicly available cancer microarray datasets with clinical information. We used this PrognoScan database to analyze the prognostic value of YAP1 in various cancers based on the hazard ratio (HR) and log-rank p-values.
- Tumor Immune Estimation Resource Database Analysis
- The Tumor Immune Estimation Resource (TIMER) database (https://cistrome.shinyapps.io/timer/) for systematic analysis was used to explore gene correlations in various cancers. The TIMER database consists of 10,897 samples across 32 cancer types from TCGA to estimate the relationship of cancer signaling pathway genes. Spearman correlation analysis of these samples was performed to determine the relationship between YAP1 expression and telomerase (TERT and TERC) [35].
- Statistical Analysis
- Gene expression data from the GEPIA were explored with online tools. Survival curves were generated with GEPIA and PrognoScan online tools. The correlations of gene expression were evaluated in the TIMER database using Spearman correlation analysis. All results are presented with p-values from the log-rank test. Statistical significance of the data (p-values) was provided by the program.
Materials and Methods
- mRNA Expression Levels of YAP1 in Various Types of Cancer
- To determine differences in YAP1 expression between tumor and normal tissue, YAP1 expression in normal samples and multiple cancer types was analyzed using the GEPIA database. The mRNA expression levels of YAP1 were higher in cholangiocarcinoma (CHOL), lymphoid neoplasm diffuse large B-cell lymphoma (DLBC), glioblastoma multiforme (GBM), pancreatic adenocarcinoma (PAAD), stomach adenocarcinoma, and thymoma (THYM) than in non-tumor tissues (Figure 1A). However, the mRNA expression levels of YAP1 were lower in adrenocortical carcinoma (ACC), bladder urothelial carcinoma (BLCA), cervical squamous cell carcinoma, pheochromocytoma and paraganglioma, uterine corpus endometrial carcinoma, and uterine carcinosarcoma than in non-tumor tissues (Figure 1B). These results suggested that YAP1 was differentially expressed between tissue samples of various cancers and non-tumor tissues.
- Prognostic Significance of YAP1 Expression in Various Types of Cancer
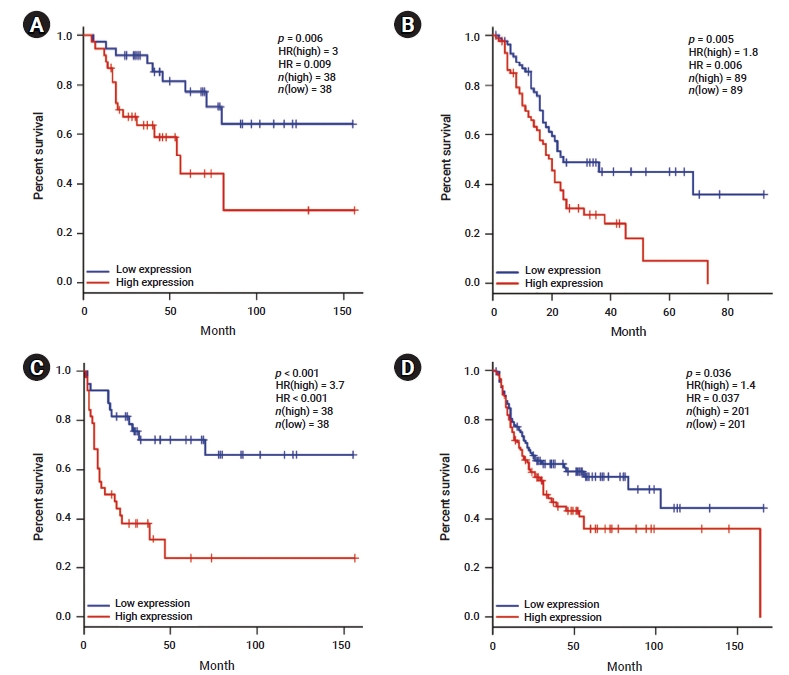
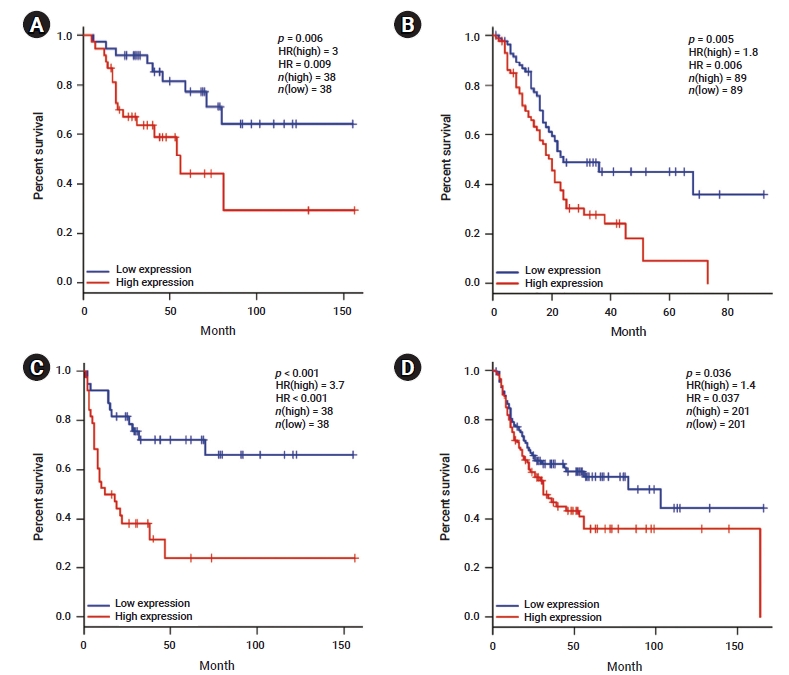
- We investigated whether YAP1 expression was correlated with the prognosis in various cancer types. Therefore, the effect of YAP1 expression on survival rates was evaluated using the GEPIA and PrognoScan databases. The OS rates of patients with different types of cancers that overexpressed or underexpressed YAP1 were compared. The results revealed shorter OS with a worse prognosis in patients with high YAP1 expression than in those with ACC (HR, 0.009; p=0.006) and PAAD (HR, 0.006; p=0.005) who had low YAP1 expression (Figure 2A, B). Moreover, the DFS rates between patients with low and high YAP1 expression were compared. High YAP1 expression was associated with poorer DFS in patients with ACC (HR, 0.000; p=0.000) and BLCA (HR, 0.037; p=0.036) (Figure 2C, D). In other cancer types, YAP1 did not have any prognostic value (Table S1). To further examine the prognostic potential of YAP1 in different cancer types, we analyzed the PrognoScan database. The analysis indicated a worse prognosis in cancers of the bladder, brain, breast, colorectal, esophagus, and lung (Table S2). These results suggest that YAP1 expression affects cancer prognosis.
- Correlation between YAP1 Expression and Telomerase in Various Types of Cancer
- To determine the correlation between YAP1 expression and TERT and TERC, we analyzed the data included in the TIMER database. As shown in Table 1, the analysis indicated YAP1 expression was negatively correlated with TERT in BLCA, breast invasive carcinoma (BRCA), CHOL, colon adenocarcinoma (COAD), brain lower grade glioma (LGG), lung adenocarcinoma (LUAD,), mesothelioma (MESO), prostate adenocarcinoma (PRAD), rectal adenocarcinoma (READ), sarcoma (SARC), testicular germ cell tumors (TGCT), thyroid carcinoma (THCA), and THYM. Moreover, YAP1 expression was negatively correlated with TERC in BLCA, BRCA, DLBC, GBM, head and neck squamous cell carcinoma (HNSC), LUAD, lung squamous cell carcinoma (LUSC), MESO, ovarian serous cystadenocarcinoma (OV), PRAD, READ, TGCT and THYM (Table 2). However, YAP1 expression was positively correlated with TERT activity in OV and uveal melanoma (UVM). These results suggested that YAP1 expression was correlated with TERT and TERC in different cancer types.
Results
- In the past decade, previous studies have focused on determining YAP1 expression to improve the understanding of its prognostic significance and potential effect on various cancer types. YAP1 is a potent oncogene [24], and its levels are frequently increased in many cancer types [1,14,36−38]. The expression and role of YAP1 in cancer are cell type-dependent, and its expression may contribute to cancer development [2,6,9,39]. Upregulation of YAP1 expression has been observed in multiple cancer types. YAP1 overexpression has been reported in patients with hepatocellular carcinoma (HCC), colorectal cancers, LUAD, ovarian cancer, and prostate cancer [2,3,14,40]. These findings suggest the potential oncogenic role of YAP1 in multiple cancer types.
- Zhang et al. [41] performed the immunohistochemical analyzes of primary esophageal squamous cell carcinoma tumor resection samples from patients, and reported that overexpression of YAP1 was associated with tumor relative to adjacent tissue samples. In addition, Collak et al. [42] identified overexpression of YAP1 in nuclear and cytosolic of benign prostates using immunohistochemistry, whereas moderate expression of YAP1 was found in cellular locations of prostate intraepithelial neoplasia and prostate cancer. These findings show differences in expression levels of YAP1 across cancer tissue samples, and are consistent with those presented in this study.
- In this study, we showed that the prognostic value of YAP1 expression was significant in various cancer types. Importantly, our data provide evidence that YAP1 expression is correlated with telomerase (TERT and TERC) expression in various cancer types. Previous studies have reported that YAP1 is a prognostic marker for OS and DFS in HCC [2]. YAP1 expression is a remarkable predictor of poor prognosis in HCC patients with negative keratin 19 cells [9]. YAP1 expression has also been significantly correlated with a poor prognosis in OV [6,36,43,44]. However, our results showed that YAP1 expression did not have a prognostic role in various cancers, including HCC and OSC, based on TCGA data. Interestingly, higher expression of YAP1 predicted poorer OS in patients with ACC and PAAD, and poorer DFS in patients with ACC and BLCA. According to these results, we suggest that higher expression of YAP1 may be significantly correlated to a poorer prognosis in various cancers.
- Telomerase is active in adult germ-line tissues, immortal cells [18], and most types of malignant tumors [19]. It is upregulated during tumorigenesis through the transcriptional regulation of TERT in up to 90% of cancers [45−48]. Upregulation of TERC is an early event in tumorigenesis, and TERC could be more closely correlated with tumor grade than telomerase activity or TERT expression [49−55]. TERC activity is associated with cancer, but its underlying mechanisms are poorly understood. This is the first study to explore the correlations between YAP1 expression and telomerase expression (TERT and TERC) in various cancer types. Our data indicated that YAP1 expression was negatively correlated with TERT in BLCA, BRCA, CHOL, COAD, LGG, MESO, PRAD, READ, SARC, TGCT, THCA, and THYM. YAP1 expression was positively correlated with TERT in OV and UVM, but negatively correlated with TERC in BLCA, BRCA, DLBC, GBM, HNSC, LIHC, LUSC, MESO, OV, PRAD, READ, TGCT, and THYM. Our findings may suggest that YAP1 expression affects TERT and TERC expression in different cancer types. However, further investigation should be performed to elucidate the potential role of YAP1 and telomere-related gene expression, which may contribute to novel research and therapies for treating various cancer types. Our results indicate that YAP1 expression is correlated with 2 major components of telomerase, and is associated with a poor prognosis in various cancer types.
- In this study, we attempted to confirm the clinical value of YAP1 expression in various cancer types and its effect on telomerase. Although YAP1 expression was different in many cancer types, the detailed mechanism underlying YAP1 regulation should be established in future studies.
Discussion
- We suggest that YAP1 could be a potential prognostic biomarker, which may stimulate novel cancer research. Understanding the correlation between YAP1 expression and telomerase may provide insights into telomere-related diseases, including different types of cancers. Therefore, future oncology research could seek to understand the biological functions of YAP1 and the correlation between YAP1 and telomere-associated gene expression.
Conclusion
-
Ethics Approval
Not applicable.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
This research was supported by the National Research Foundation Grant funded by the Korean Government (NRF-2021R1C1C1003333 to HSJ).
-
Availability of Data
All data generated or analyzed during this study are included in this published article. Other data may be requested through the corresponding author.
-
Authors’ Contributions
Conceptualization: SJH, JK; Data curation: SJH, JK; Formal analysis: SJH, JK; Funding acquisition: SJH; Investigation: HRK, KY, CWS; Methodology: HRK, KY, CWS; Project administration: HRK, KY, CWS; Resources: HRK, KY, CWS; Software: HRK, KY, CWS; Supervision: HRK, KY, CWS; Validation: HRK, KY; Visualization: HRK, KY; Writing–original draft: all authors; Writing–review & editing: all authors.
Article information
Supplementary Material
Table S1.
Table S2.

| Cancer type | R | p |
|---|---|---|
| Adrenocortical carcinoma | 0.10 | 0.384 |
| Bladder urothelial carcinoma | –0.14 | 0.005* |
| Breast invasive carcinoma | –0.14 | 0.000* |
| Cervical squamous cell carcinoma and endocervical adenocarcinoma | 0.03 | 0.547 |
| Cholangiocarcinoma | –0.35 | 0.038* |
| Colon adenocarcinoma | –0.12 | 0.012* |
| Lymphoid neoplasm diffuse large B-cell lymphoma | 0.05 | 0.750 |
| Esophageal carcinoma | 0.03 | 0.729 |
| Glioblastoma multiforme | 0.01 | 0.908 |
| Head and neck squamous cell carcinoma | –0.04 | 0.326 |
| Kidney chromophobe | 0.07 | 0.604 |
| Kidney renal clear cell carcinoma | –0.08 | 0.062 |
| Kidney renal papillary cell carcinoma | 0.04 | 0.509 |
| Brain lower grade glioma | –0.28 | 0.000* |
| Liver hepatocellular carcinoma | –0.08 | 0.142 |
| Lung adenocarcinoma | –0.09 | 0.045* |
| Lung squamous cell carcinoma | 0.01 | 0.839 |
| Mesothelioma | –0.25 | 0.022* |
| Ovarian serous cystadenocarcinoma | 0.20 | 0.000* |
| Pancreatic adenocarcinoma | –0.08 | 0.298 |
| Pheochromocytoma and paraganglioma | –0.04 | 0.636 |
| Prostate adenocarcinoma | –0.24 | 0.000* |
| Rectum adenocarcinoma | –0.29 | 0.000* |
| Sarcoma | –0.22 | 0.000* |
| Skin cutaneous melanoma | 0.04 | 0.397 |
| Stomach adenocarcinoma | –0.08 | 0.100 |
| Testicular germ cell tumors | –0.18 | 0.031* |
| Thyroid carcinoma | –0.13 | 0.004* |
| Thymoma | –0.50 | 0.000* |
| Uterine corpus endometrial carcinoma | 0.01 | 0.760 |
| Uterine carcinosarcoma | 0.08 | 0.553 |
| Uveal melanoma | 0.31 | 0.005* |
| Cancer type | R | p |
|---|---|---|
| Adrenocortical carcinoma | –0.07 | 0.568 |
| Bladder urothelial carcinoma | –0.14 | 0.005* |
| Breast invasive carcinoma | –0.18 | 0.000* |
| Cervical squamous cell carcinoma and | –0.03 | 0.587 |
| endocervical adenocarcinoma | ||
| Cholangiocarcinoma | 0.06 | 0.742 |
| Colon adenocarcinoma | –0.03 | 0.468 |
| Lymphoid neoplasm diffuse large B-cell lymphoma | –0.31 | 0.035* |
| Esophageal carcinoma | –0.07 | 0.336 |
| Glioblastoma multiforme | –0.18 | 0.023* |
| Head and neck squamous cell carcinoma | –0.11 | 0.016* |
| Kidney chromophobe | 0.18 | 0.156 |
| Kidney renal clear cell carcinoma | –0.07 | 0.101 |
| Kidney renal papillary cell carcinoma | –0.07 | 0.243 |
| Brain lower grade glioma | –0.01 | 0.748 |
| Liver hepatocellular carcinoma | 0.05 | 0.345 |
| Lung adenocarcinoma | –0.16 | 0.000* |
| Lung squamous cell carcinoma | –0.12 | 0.007* |
| Mesothelioma | –0.28 | 0.007* |
| Ovarian serous cystadenocarcinoma | –0.14 | 0.012* |
| Pancreatic adenocarcinoma | –0.12 | 0.122 |
| Pheochromocytoma and paraganglioma | –0.05 | 0.484 |
| Prostate adenocarcinoma | –0.18 | 0.000* |
| Rectum adenocarcinoma | –0.39 | 0.000* |
| Sarcoma | –0.09 | 0.144 |
| Skin cutaneous melanoma | –0.03 | 0.588 |
| Stomach adenocarcinoma | –0.07 | 0.173 |
| Testicular germ cell tumors | –0.23 | 0.005* |
| Thyroid carcinoma | –0.04 | 0.381 |
| Thymoma | –0.56 | 0.000* |
| Uterine corpus endometrial carcinoma | 0.08 | 0.071 |
| Uterine carcinosarcoma | 0.16 | 0.236 |
| Uveal melanoma | –0.05 | 0.642 |
- 1. Wang Y, Dong Q, Zhang Q, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci 2010;101:1279−85.ArticlePubMed
- 2. Xu MZ, Yao TJ, Lee NP, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 2009;115:4576−85.ArticlePubMed
- 3. Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007;21:2747−61.ArticlePubMedPMC
- 4. Orr BA, Bai H, Odia Y, et al. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol 2011;70:568−77.ArticlePubMed
- 5. Pan D. The hippo signaling pathway in development and cancer. Dev Cell 2010;19:491−505.ArticlePubMedPMC
- 6. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 2013;13:246−57.ArticlePubMed
- 7. Zhou Q, Bauden M, Andersson R, et al. YAP1 is an independent prognostic marker in pancreatic cancer and associated with extracellular matrix remodeling. J Transl Med 2020;18:77. ArticlePubMedPMC
- 8. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 2011;13:877−83.ArticlePubMedPMC
- 9. Van Haele M, Moya IM, Karaman R, et al. YAP and TAZ heterogeneity in primary liver cancer: an analysis of its prognostic and diagnostic role. Int J Mol Sci 2019;20:638. ArticlePubMedPMC
- 10. Lamar JM, Stern P, Liu H, et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A 2012;109:E2441−50.ArticlePubMedPMC
- 11. Guo L, Chen Y, Luo J, et al. YAP1 overexpression is associated with poor prognosis of breast cancer patients and induces breast cancer cell growth by inhibiting PTEN. FEBS Open Bio 2019;9:437−45.ArticlePubMedPMC
- 12. Werneburg N, Gores GJ, Smoot RL. The Hippo pathway and YAP signaling: emerging concepts in regulation, signaling, and experimental targeting strategies with implications for hepatobiliary malignancies. Gene Expr 2020;20:67−74.ArticlePubMedPMC
- 13. Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer 2012;48:1227−34.ArticlePubMed
- 14. Kang W, Tong JH, Chan AW, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res 2011;17:2130−9.ArticlePubMed
- 15. Pestana A, Vinagre J, Sobrinho-Simoes M, et al. TERT biology and function in cancer: beyond immortalisation. J Mol Endocrinol 2017;58:R129−46.ArticlePubMed
- 16. Lingner J, Cech TR. Purification of telomerase from Euplotes aediculatus: requirement of a primer 3' overhang. Proc Natl Acad Sci U S A 1996;93:10712−7.ArticlePubMedPMC
- 17. Li C, Wu MY, Liang YR, et al. Correlation between expression of human telomerase subunits and telomerase activity in esophageal squamous cell carcinoma. World J Gastroenterol 2003;9:2395−9.ArticlePubMedPMC
- 18. Shen ZY, Xu LY, Li EM, et al. Telomere and telomerase in the initial stage of immortalization of esophageal epithelial cell. World J Gastroenterol 2002;8:357−62.ArticlePubMedPMC
- 19. Hiyama E, Hiyama K. Clinical utility of telomerase in cancer. Oncogene 2002;21:643−9.ArticlePubMed
- 20. Liu Z, Li Q, Li K, et al. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene 2013;32:4203−13.ArticlePubMed
- 21. Takakura M, Kyo S, Kanaya T, et al. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res 1999;59:551−7.PubMed
- 22. Reddel RR. Telomere maintenance mechanisms in cancer: clinical implications. Curr Pharm Des 2014;20:6361−74.ArticlePubMedPMC
- 23. Zhang Q, Liu N, Bai J, et al. Human telomerase reverse transcriptase is a novel target of Hippo-YAP pathway. FASEB J 2020;34:4178−88.ArticlePubMed
- 24. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 2015;15:73−9.ArticlePubMedPMC
- 25. Jung SJ, Seo YR, Park WJ, et al. Clinicopathological characteristics of TZAP expression in colorectal cancers. Onco Targets Ther 2020;13:12933−42.PubMedPMC
- 26. Chiang YJ, Hemann MT, Hathcock KS, et al. Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo. Mol Cell Biol 2004;24:7024−31.ArticlePubMedPMC
- 27. Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J 2006;25:565−74.ArticlePubMedPMC
- 28. Cayuela ML, Flores JM, Blasco MA. The telomerase RNA component Terc is required for the tumour-promoting effects of Tert overexpression. EMBO Rep 2005;6:268−74.ArticlePubMedPMC
- 29. Soder AI, Going JJ, Kaye SB, et al. Tumour specific regulation of telomerase RNA gene expression visualized by in situ hybridization. Oncogene 1998;16:979−83.ArticlePubMed
- 30. Kedde M, le Sage C, Duursma A, et al. Telomerase-independent regulation of ATR by human telomerase RNA. J Biol Chem 2006;281:40503−14.ArticlePubMed
- 31. Chen B, Lai J, Dai D, et al. JAK1 as a prognostic marker and its correlation with immune infiltrates in breast cancer. Aging (Albany NY) 2019;11:11124−35.ArticlePubMedPMC
- 32. Gu Y, Li X, Bi Y, et al. CCL14 is a prognostic biomarker and correlates with immune infiltrates in hepatocellular carcinoma. Aging (Albany NY) 2020;12:784−807.ArticlePubMedPMC
- 33. Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98−102.ArticlePubMedPMC
- 34. Mizuno H, Kitada K, Nakai K, et al. PrognoScan: a new database for meta-analysis of the prognostic value of genes. BMC Med Genomics 2009;2:18. ArticlePubMedPMC
- 35. Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res 2017;77:e108−10.ArticlePubMedPMC
- 36. Hall CA, Wang R, Miao J, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res 2010;70:8517−25.ArticlePubMedPMC
- 37. Liu T, Liu Y, Gao H, et al. Clinical significance of yes-associated protein overexpression in cervical carcinoma: the differential effects based on histotypes. Int J Gynecol Cancer 2013;23:735−42.PubMed
- 38. Zhang J, Xu ZP, Yang YC, et al. Expression of Yes-associated protein in gastric adenocarcinoma and inhibitory effects of its knockdown on gastric cancer cell proliferation and metastasis. Int J Immunopathol Pharmacol 2012;25:583−90.ArticlePubMed
- 39. Park J, Eisenbarth D, Choi W, et al. YAP and AP-1 cooperate to initiate pancreatic cancer development from ductal cells in mice. Cancer Res 2020;80:4768−79.ArticlePubMed
- 40. Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol 2008;39:1582−9.ArticlePubMedPMC
- 41. Zhang Q, Lou L, Cai X, et al. Clinical significance of AJUBA, YAP1, and MMP14 expression in esophageal squamous cell carcinoma. Int J Clin Exp Pathol 2018;11:6018−24.PubMedPMC
- 42. Collak FK, Demir U, Ozkanli S, et al. Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension. Cancer Biol Med 2017;14:405−13.ArticlePubMedPMC
- 43. Cho SY, Kim K, Park MS, et al. Expression of Yes-associated protein 1 and its clinical significance in ovarian serous cystadenocarcinoma. Oncol Rep 2017;37:2620−32.ArticlePubMedPMC
- 44. Zhang X, George J, Deb S, et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011;30:2810−22.ArticlePubMed
- 45. Peters B. Response to Letter to the Editor "Hyponatremia may be an under-recognised complication after desmopressin to reduce uremic bleeding in kidney biopsy". Nephrology (Carlton) 2020;25:584. ArticlePubMed
- 46. Yuan X, Larsson C, Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players. Oncogene 2019;38:6172−83.ArticlePubMedPMC
- 47. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer 1997;33:787−91.ArticlePubMed
- 48. Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev 2002;66:407−25.ArticlePubMedPMC
- 49. Soder AI, Hoare SF, Muir S, et al. Amplification, increased dosage and in situ expression of the telomerase RNA gene in human cancer. Oncogene 1997;14:1013−21.ArticlePubMed
- 50. Brown DF, Gazdar AF, White CL 3rd, et al. Human telomerase RNA expression and MIB-1 (Ki-67) proliferation index distinguish hemangioblastomas from metastatic renal cell carcinomas. J Neuropathol Exp Neurol 1997;56:1349−55.ArticlePubMed
- 51. Morales CP, Lee EL, Shay JW. In situ hybridization for the detection of telomerase RNA in the progression from Barrett's esophagus to esophageal adenocarcinoma. Cancer 1998;83:652−9.ArticlePubMed
- 52. Maitra A, Yashima K, Rathi A, et al. The RNA component of telomerase as a marker of biologic potential and clinical outcome in childhood neuroblastic tumors. Cancer 1999;85:741−9.ArticlePubMed
- 53. Yashima K, Litzky LA, Kaiser L, et al. Telomerase expression in respiratory epithelium during the multistage pathogenesis of lung carcinomas. Cancer Res 1997;57:2373−7.PubMed
- 54. Yi X, Tesmer VM, Savre-Train I, et al. Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol Cell Biol 1999;19:3989−97.ArticlePubMedPMC
- 55. Dome JS, Bockhold CA, Li SM, et al. High telomerase RNA expression level is an adverse prognostic factor for favorable-histology Wilms' tumor. J Clin Oncol 2005;23:9138−45.ArticlePubMed
References
Figure & Data
References
Citations



 PubReader
PubReader ePub Link
ePub Link Cite
Cite