Search
- Page Path
- HOME > Search
- Points to consider for COVID-19 vaccine quality control and national lot release in Republic of Korea: focus on a viral vector platform
- Jung Hun Ju, Naery Lee, Sun-hee Kim, Seokkee Chang, Misook Yang, Jihyun Shin, Eunjo Lee, Sunhwa Sung, Jung-Hwan Kim, Jin Tae Hong, Ho Jung Oh
- Osong Public Health Res Perspect. 2022;13(1):4-14. Published online February 8, 2022
- DOI: https://doi.org/10.24171/j.phrp.2021.0311
- 5,790 View
- 169 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 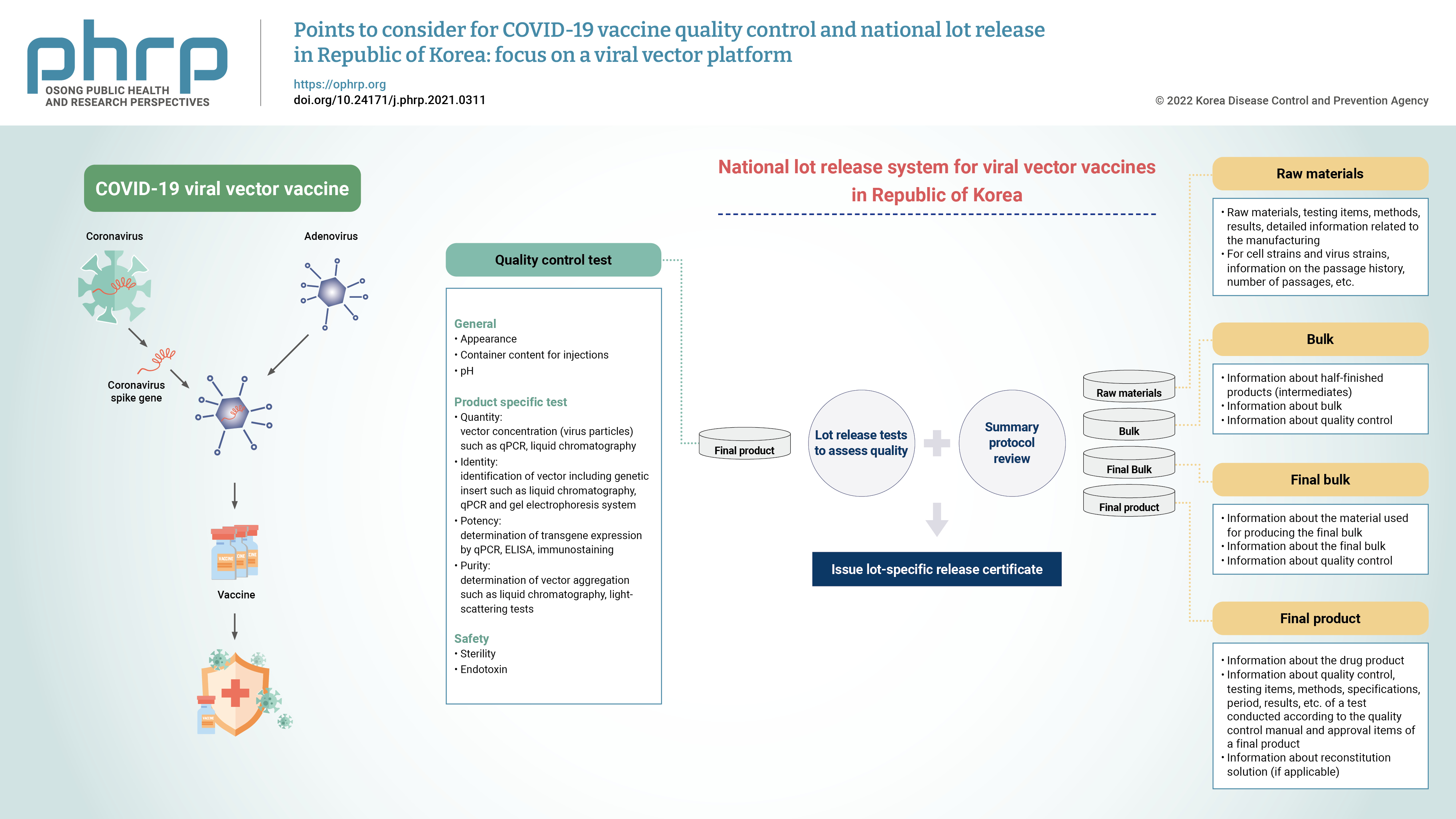
- Due to the global public health crisis caused by the coronavirus disease 2019 (COVID-19) pandemic, the importance of vaccine development has increased. In particular, a rapid supply of vaccines and prompt deployment of vaccination programs are essential to prevent and overcome the spread of COVID-19. As a part of the vaccine regulations, national lot release is regulated by the responsible authorities, and this process involves the assessment of the lot before a vaccine is marketed. A lot can be released for use when both summary protocol (SP) review and quality control testing are complete. Accelerated lot release is required to distribute COVID-19 vaccines in a timely manner. In order to expedite the process by simultaneously undertaking the verification of quality assessment and application for approval, it is necessary to prepare the test methods before marketing authorization. With the prolonged pandemic and controversies regarding the effectiveness of the COVID-19 vaccine against new variants, public interest for the development of a new vaccine are increasing. Domestic developers have raised the need to establish standard guidance on the requirements for developing COVID-19 vaccine. This paper presents considerations for quality control in the manufacturing process, test items, and SP content of viral vector vaccines.
- The 2nd Meeting of National Control Laboratories for Vaccines and Biologicals in the Western Pacific
- Hokyung Oh, Jinho Shin, Chung Keel Lee, Masaki Ochiai, Kiyoko Nojima, Chang Kweng Lim, Sanj Raut, Irene Lisovsky, Stella Williams, Ki Young Yoo, Dong-Yeop Shin, Manabu Ato, Qiang Ye, Kiwon Han, Chulhyun Lee, Naery Lee, Ji Young Hong, Kikyung Jung, Pham Van Hung, Jayoung Jeong
- Osong Public Health Res Perspect. 2018;9(3):133-139. Published online June 30, 2018
- DOI: https://doi.org/10.24171/j.phrp.2018.9.3.10
- 4,246 View
- 107 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF The Second Meeting of the National Control Laboratories for Vaccines and Biologicals in the Western Pacific, was jointly organized by the National Institute of Food and Drug Safety Evaluation of the Ministry of Food and Drug Safety in the Republic of Korea, and by the World Health Organization Regional Office for the Western Pacific.
In the National Lot Release Systems session countries including Canada, China, Japan, Malaysia, Vietnam, and the Republic of Korea, all shared information on their current Lot Release Systems, including current practices and developments in risk-based official lot release of vaccines.
In the session on Quality Control of Blood Products, experts from the National Institute for Biological Standards and Control shared quality control and research results for; blood coagulation factor VIII products, and the measurement of procoagulant activity in immunoglobulin products. Representatives from Japan proposed a regional collaborative study to test aggregated immunoglobulin free from complement activity. A cell-based Japanese encephalitis vaccine potency assay was proposed by representatives from Korea and they also called for voluntary participation of other National Control Laboratories in a collaborative study, on the first Korean
Gloydius anti-venom standard. Participants agreed in general to continue communicating, and coordinate presentation of the study results.-
Citations
Citations to this article as recorded by- A collaborative study to establish the second national standard for hepatitis B immunoglobulin in Korea
Chan Woong Choi, Su Kyoung Seong, Ki Won Han, Hyun Jeong Kim, Kyung Hee Sohn, Sun Bo Shim, Yun Su Bang, JungHwan Cho, In Soo Shin
Biologicals.2023; 82: 101679. CrossRef - Report on the seventh meeting of national control laboratories for vaccines and biologicals of the WHO Western Pacific and South-East Asia member states
Sun Bo Shim, Chan Woong Choi, Jin Ho Shin, Jong Won Kim, Silke Schepelmann, Jae Ho Jung, Harish Chander, Ratih Pujilestari, Madoka Kuramitsu, Masaki Ochiai, Nee Yuan Qi, Geraldine N. Dimapilis, Luu Thi Dung, Hyung Sil Moon, In Soo Shin
Biologicals.2023; 84: 101712. CrossRef
- A collaborative study to establish the second national standard for hepatitis B immunoglobulin in Korea
- National Biobank of Korea: Quality control Programs of Collected-human Biospecimens
- Jae-Eun Lee, Ji-Hyun Kim, Eun-Jung Hong, Hye Sook Yoo, Hye-Young Nam, Ok Park
- Osong Public Health Res Perspect. 2012;3(3):185-189. Published online June 30, 2012
- DOI: https://doi.org/10.1016/j.phrp.2012.07.007
- 2,863 View
- 16 Download
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF - Personalized medicine is emerging as a main paradigm for risk prediction, pre-diagnosis, and effective prevention and treatment of disease. A large number of human biospecimens and their clinical data are essential resources for the success of personalized medicine as well as other biomedical research. The National Biobank of Korea (NBK) has collected well-annotated and high quality human biospecimens, and distributes them to the Korean biomedical scientists, through the Korea Biobank Project (KBP). The ultimate goal of NBK activities is to promote biomedical research and public health. As of December- 2011, the NBK has collected various human biospecimens from 525,416 participants including 325,952 Korean populations and 199,464 patients. The purpose of this paper is to introduce the KBP and quality control programs for collection of human biospecimens with high quality of NBK.
-
Citations
Citations to this article as recorded by- Evaluation of penalized and machine learning methods for asthma disease prediction in the Korean Genome and Epidemiology Study (KoGES)
Yongjun Choi, Junho Cha, Sungkyoung Choi
BMC Bioinformatics.2024;[Epub] CrossRef - Urinary microbiome-based metagenomic signature for the noninvasive diagnosis of hepatocellular carcinoma
Eun Ju Cho, Boram Kim, Su Jong Yu, Suk Kyun Hong, YoungRok Choi, Nam-Joon Yi, Kwang-Woong Lee, Kyung-Suk Suh, Jung-Hwan Yoon, Taesung Park
British Journal of Cancer.2024; 130(6): 970. CrossRef - Assessment of the Impact of Preanalytical DNA Integrity on the Genome Data Quality
Sung-Mi Shim, Meehee Lee, Jae-Pil Jeon
Biopreservation and Biobanking.2024;[Epub] CrossRef - Gene–Smoking Interaction Analysis for the Identification of Novel Asthma-Associated Genetic Factors
Junho Cha, Sungkyoung Choi
International Journal of Molecular Sciences.2023; 24(15): 12266. CrossRef - Multi-omics reveals microbiome, host gene expression, and immune landscape in gastric carcinogenesis
Chan Hyuk Park, Changjin Hong, A-reum Lee, Jaeyun Sung, Tae Hyun Hwang
iScience.2022; 25(3): 103956. CrossRef - Biobank for multidrug-resistant tuberculosis research: importance of sequential samples
Yoohyun Hwang, Jiyeon Kim, Seungkyu Park, Sungweon Ryoo
Pathogens and Disease.2021;[Epub] CrossRef - Comparison between Cervical Ureaplasma spp. Colonization and the Intensity of Inflammatory Mediators in the Amniotic Fluid Retrieved during Cesarean Delivery in Preterm Birth
Jingon Bae, Shin Kim, Ilseon Hwang, Jaehyun Park
International Journal of Environmental Research an.2021; 19(1): 107. CrossRef - Contributions of the UK biobank high impact papers in the era of precision medicine
Peter Glynn, Philip Greenland
European Journal of Epidemiology.2020; 35(1): 5. CrossRef - Axes of a revolution: challenges and promises of big data in healthcare
Smadar Shilo, Hagai Rossman, Eran Segal
Nature Medicine.2020; 26(1): 29. CrossRef - Is the Random Forest Algorithm Suitable for Predicting Parkinson’s Disease with Mild Cognitive Impairment out of Parkinson’s Disease with Normal Cognition?
Haewon Byeon
International Journal of Environmental Research an.2020; 17(7): 2594. CrossRef - Application of Machine Learning Technique to Distinguish Parkinson’s Disease Dementia and Alzheimer’s Dementia: Predictive Power of Parkinson’s Disease-Related Non-Motor Symptoms and Neuropsychological Profile
Haewon Byeon
Journal of Personalized Medicine.2020; 10(2): 31. CrossRef - Exploring the Predictors of Rapid Eye Movement Sleep Behavior Disorder for Parkinson’s Disease Patients Using Classifier Ensemble
Haewon Byeon
Healthcare.2020; 8(2): 121. CrossRef - DeepVariant-on-Spark: Small-Scale Genome Analysis Using a Cloud-Based Computing Framework
Po-Jung Huang, Jui-Huan Chang, Hou-Hsien Lin, Yu-Xuan Li, Chi-Ching Lee, Chung-Tsai Su, Yun-Lung Li, Ming-Tai Chang, Sid Weng, Wei-Hung Cheng, Cheng-Hsun Chiu, Petrus Tang
Computational and Mathematical Methods in Medicine.2020; 2020: 1. CrossRef - Mini-Review of Laboratory Operations in Biobanking: Building Biobanking Resources for Translational Research
Mine S. Cicek, Janet E. Olson
Frontiers in Public Health.2020;[Epub] CrossRef - Development of a depression in Parkinson's disease prediction model using machine learning
Haewon Byeon
World Journal of Psychiatry.2020; 10(10): 234. CrossRef - Aspects of Modern Biobank Activity – Comprehensive Review
Wiktor Paskal, Adriana M. Paskal, Tomasz Dębski, Maciej Gryziak, Janusz Jaworowski
Pathology & Oncology Research.2018; 24(4): 771. CrossRef - Optimization of RNA Extraction from Formalin-Fixed Paraffin-Embedded Blocks for Targeted Next-Generation Sequencing
Yoojin Choi, Aeree Kim, Jinkyoung Kim, Jinhwan Lee, Soo Yeon Lee, Chungyeul Kim
Journal of Breast Cancer.2017; 20(4): 393. CrossRef - Biobank Regulation in South Korea
Won Bok Lee
Journal of Law, Medicine & Ethics.2016; 44(2): 342. CrossRef - Standard based Deposit Guideline for Distribution of Human Biological Materials in Cancer Patients
Hwa Jeong Seo, Hye Hyeon Kim, Jeong Soo Im, Ju Han Kim
Asian Pacific Journal of Cancer Prevention.2014; 15(14): 5545. CrossRef - Basis for Korean Genome Study
Hae-Wol Cho, Chaeshin Chu
Osong Public Health and Research Perspectives.2012; 3(3): 119. CrossRef
- Evaluation of penalized and machine learning methods for asthma disease prediction in the Korean Genome and Epidemiology Study (KoGES)



 First
First Prev
Prev


