Prevalence and patterns of post-COVID-19 symptoms in recovered patients of Delhi, India: a population-based study
Article information
Abstract
Objectives
Post-coronavirus disease 2019 (COVID-19) symptoms were widely reported.However, data on post-COVID-19 conditions following infection with the Omicron variant remained scarce. This prospective study was conducted to understand the prevalence, patterns, and duration of symptoms in patients who had recovered from COVID-19.
Methods
A prospective study was conducted across 11 districts of Delhi, India, among individuals who had recovered from COVID-19. Study participants were enrolled, and then returned for post-recovery follow-up at 3 months and 6 months interval.
Results
The mean age of study participants was 42.07 years, with a standard deviation of 14.89 years. The majority of the participants (79.7%) reported experiencing post-COVID-19 symptoms. The most common symptoms included joint pain (36%), persistent dry cough (35.7%), anxiety (28.4%), and shortness of breath (27.1%). Other symptoms were persistent fatigue (21.6%), persistent headache (20%), forgetfulness (19.7%), and limb weakness (18.6%). The longest duration of symptom was observed to be anxiety (138.75±54.14 days), followed by fatigue (137.57±48.33 days), shortness of breath (131.89±60.21 days), and joint pain/swelling (131.59±58.76 days). At the first follow-up visit, 2.2% of participants presented with abnormal electrocardiogram readings, but no abnormalities were noticed during the second follow-up. Additionally, 4.06% of participants exhibited abnormal chest X-ray findings at the first follow-up, which decreased to 2.16% by the second visit.
Conclusion
The most frequently reported post-COVID-19 symptoms were joint pain, dry cough, anxiety and shortness of breath. These clinical symptoms persisted for up to 6 months, with evidence of multi-system involvement. Consequently, findings highlighted the need for long-term follow-up during the post-COVID-19 period.
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first spotted in China in December 2019, led to a global pandemic. This pandemic led to a rapid increase in both cases and deaths, as well as overwhelming healthcare infrastructure [1]. On May 5, 2023, the World Health Organization (WHO) announced the end of COVID-19 as a public health emergency of international concern, with over 768 million cases and more than 6.9 million deaths reported to date [2]. The pandemic spurred numerous epidemiological studies aimed at understanding the transmission of the disease, as well as prevention and control strategies. The variability of the disease—influenced by the circulating viral strains—and a limited understanding of its pathophysiology led to the exploration of multiple treatment options during the peak of the outbreak. The broad clinical spectrum of SARS-CoV-2 further complicated the understanding of the disease due to its potential for multiorgan involvement [3,4].
Acute COVID-19 infection reportedly lasts up to 4 weeks before recovery [5]. However, growing evidence suggested that signs and symptoms of COVID-19 infection persist even after recovery in a substantial proportion of cases. The condition characterized by the persistence of symptoms after the initial infection and recovery from COVID-19 has been termed “long COVID,” “post-COVID-19 syndrome,” and “post-acute COVID-19 syndrome” [5]. No consensus has yet been reached on the characteristics defining this condition. The British Medical Association defines a syndrome as a “set of medical signs and symptoms which are correlated with each other and associated with a particular disease” [6]. More specifically, the UK National Institute of Health and Care Excellence defines post-COVID-19 syndrome as “a cluster of signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis” [5]. Long-term symptoms of COVID-19 can include cardiopulmonary symptoms (a decline in respiratory function, fibrosis, pleural involvement, myocarditis, pericardial effusion, etc.), post-COVID-19 thrombosis, immune-mediated manifestations (arthritis, myositis, pancreatitis, etc.), and other skin, neurological, renal, hematological, endocrine, and systemic manifestations [5,7]. The presence of persistent COVID-19 symptoms has reportedly significantly affected the mental health and emotional well-being of patients [8].
To date, research into post-COVID-19 syndrome has been limited, with particularly scant data on long-term outcomes among recovered patients in emerging economies [8,9].Under this backdrop, we designed a study to evaluate the post-COVID phenomenon or symptomatology in patients who have recovered from COVID-19 in Delhi, India. The aim of this research was to ascertain the prevalence, patterns, and duration of symptoms in these recovered individuals.
Materials and Methods
Study Design
A prospective observational study was conducted across 11 districts of Delhi among individuals who had recovered from COVID-19. Study participants were enrolled after recovery, then returned for follow-up at interval of 3 months and 6 months.
Study Area and Period
The study was conducted during October 2021 to July 2023, across 11 districts of Delhi, India.
Inclusion Criteria
The inclusion criteria encompassed adult individuals (aged 18 years and older) with a history of mild, moderate, or severe COVID-19 in the prior 6 months, diagnosed at least 14 days before study enrollment.
Exclusion Criteria
The study excluded pregnant women, individuals with a history of reinfection with COVID-19 and patients at the terminal stage of any disease.
Sample Size and Sampling Technique
The reported prevalence of long COVID is 22%. Given this prevalence rate, a 95% confidence level, a relative precision of 4.4%, and accounting for a 20% allowable error and a 20% loss to follow-up, the sample size was calculated to be 410 [10]. Based on the per-protocol principle, the analysis of the collected data was based on the second follow-up sample size of 369 participants.
Data Collection Procedure
The study participants were diagnosed with COVID-19 between April 2022 and September 2022. We obtained a daily list of patients with COVID-19 who had recovered—including those who had been hospitalized and those who had managed their illness through home isolation—from the Directorate General of Health Services, Government of NCT, Delhi. The data were cleaned by removing records of individuals with missing addresses or contact numbers, as well as those under 18 years old. Subsequently, participants meeting the inclusion criteria were contacted via phone calls, followed by household visits. They were enrolled in the study after providing written informed consent for prospective follow-up at baseline, 3 months, and 6 months after recovery. During participant interviews, a detailed medical history was recordrd, along with information about their past COVID-19 episode. A comprehensive history was recorded for all participants at each household visit. For the baseline assessment, we collected sociodemographic characteristics of the participants using an interview schedule. These data included age, sex, education, socioeconomic status, healthcare-seeking behavior during the COVID-19 episode, baseline comorbidity details, and treatment profile at their household. Additionally, a standardized and validated WHO case report form for post-COVID-19 conditions was used to gather participants’ information [11]. A multidisciplinary team of doctors reviewed the medical histories and performed investigations and clinical assessments of the study participants. This was done to provide recommendations for further biochemical investigations, referrals, and treatment as needed. Blood samples were collected with participant consent and tested in a government-approved laboratory. The analyses were conducted using the Vitros 5600 integrated clinical chemistry and immunoassay analyzer and the Stago STA MAX coagulation analyzer (Tables S1 and S2).
Operational Definitions
For hospitalized patients, recovery was defined as discharge from the hospital following the completion of treatment for COVID-19. For non-hospitalized patients, recovery was indicated by a period of 14 days from symptom onset and since diagnostic confirmation using reverse transcription-polymerase chain reaction (RT-PCR) or antigen tests. Acute COVID-19 encompassed symptoms experienced up to 4 weeks after the diagnosis of SARS-CoV-2 infection [5]. Ongoing symptomatic COVID-19 referred to the presence of symptoms 4 to 12 weeks following confirmed SARS-CoV-2 infection [5]. Post-COVID-19 was defined as symptoms developed during or after SARS-CoV-2 infection that persisted for more than 12 weeks [5]. Long COVID referred to signs and symptoms that continued for more than 4 weeks, encompassing both the ongoing symptomatic COVID-19 and post-COVID-19 syndrome subgroups [5]. Mild disease was defined as upper respiratory tract symptoms without shortness of breath or hypoxia [5]. Moderate disease was characterized by upper respiratory tract symptoms accompanied by shortness of breath or hypoxia, necessitating the administration of supplemental oxygen at home. These findings included a respiratory rate (RR) greater than 24 breaths per minute, breathlessness, and an oxygen saturation (SpO2) of 90% to less than 93% on room air [5]. Severe cases were those involving hospitalization with COVID-19 infection, characterized by an RR above 30 breaths per minute, breathlessness, and an SpO2 below 90% on room air [5].
Statistical Analysis
The data were entered into Excel (Microsoft) and analyzed using IBM SPSS ver. 25.0 (IBM Corp.). Results were presented as frequencies and proportions for categorical variables, median (interquartile range [IQR]) for non-normally distributed continuous data, and mean±standard deviation for normally distributed continuous data. The prevalence and incidence of post-COVID-19 symptoms and consequences were assessed at baseline and during each follow-up visit. A per-protocol analysis was employed due to the lower attrition rate in the follow-up period. Standard cut-off scores were used to evaluate the presence of depression, anxiety, poor sleep quality, and suboptimal quality of life, which constituted dependent variables. The independent variables included age, sex, severity of COVID-19, duration of hospitalization, and history of tobacco smoking and substance abuse. To compare differences in means between 2 groups, we used the Student unpaired t-test or the Mann-Whitney U test. For assessments involving more than 2 groups at the 3 time points, we conducted a repeated measures analysis of variance with the Tukey post hoc test or the Friedman test, depending on the data distribution. A p-value of less than 0.05 was considered to indicate statistical significance.
Results
The study included 413 participants, all of whom presented with mild disease. The response rate was 91.8% (379 patients) at the first follow-up visit and 89.3% (369 patients) at the second follow-up. Following the per-protocol principle, the collected data were analyzed based on the 369 patients who completed the second follow-up. The mean age of the participants was 42.07 years, with a standard deviation of 14.89 years. We examined the sociodemographic characteristics of the participants, including sex, educational status, smoking history, and alcohol consumption. Approximately half (50.1%) of the participants were male, 97.84% were literate, 92.96% had never smoked, and 9.21% reported a history of alcohol consumption. Most participants (93.5%) were vaccinated, and 26.56% had at least 1 comorbidity (Table 1). The analysis revealed significant associations between post-COVID-19 syndrome and both sex and age (p<0.05).
Nearly half of the participants (47.5%) experienced long COVID symptoms, while 79.7% had post-COVID-19 symptoms (Figure 1). The most common symptoms included joint pain (36%), persistent dry cough (35.7%), anxiety (28.4%), and shortness of breath (27.1%). Other symptoms reported were persistent fatigue (21.6%), persistent headache (20.0%), forgetfulness (19.7%), and limb weakness (18.6%) (Table 2). The longest symptom duration was reported for anxiety, at 132 days (IQR, 97–190 days), followed by fatigue at 127 days (IQR, 97–172 days), body ache at 124 days (IQR, 89–157 days), and joint pain/swelling at 115 days (IQR, 89–180 days). Prolonged symptom duration was also noted for shortness of breath, at 112.5 days (IQR, 88.25–181.50 days); headache, at 109.5 days (IQR, 87–139.50 days); and limb weakness, at 109 days (IQR, 83–148.75 days). Anxiety and persistent dry cough had significantly longer durations in men compared to women (p<0.05). Relative to the unvaccinated subgroup, limb weakness and fever lasted significantly longer in vaccinated participants (p<0.05). Additional symptoms included dry cough, lasting 105.5 days (IQR, 86–146 days), and forgetfulness, lasting 100 days (IQR, 87.5–145 days). Notably, forgetfulness persisted for significantly longer in participants with any comorbidity than in those without comorbid conditions (Table 3).
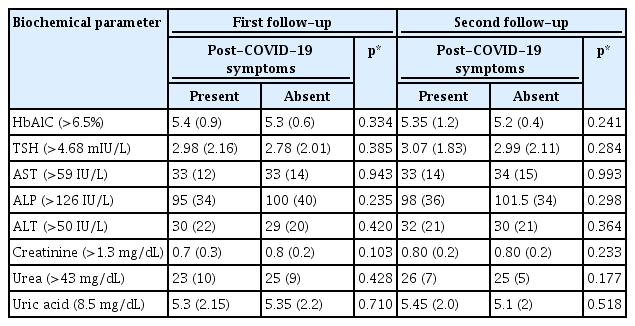
Biochemical investigations were conducted for study participants at baseline, the first follow-up, and the second follow-up. The Shapiro-Wilk normality test indicated that the data were skewed in the distribution of the biochemical parameters. The analysis revealed no significant differences in the median levels of thyroid-stimulating hormone (TSH), hemoglobin A1c (HbA1c), aspartate aminotransferase/serum glutamic oxaloacetic transaminase (AST/SGOT), alanine aminotransferase/serum glutamic pyruvic transaminase (ALT/SGPT), alkaline phosphatase (ALP), creatinine, urea, or uric acid between individuals with post-COVID-19 syndrome and those without the syndrome (p>0.05). The median values for all parameters fell within normal ranges (Table 4). New instances of elevated HbA1c were detected in 3.5% of patients at the first follow-up and in 6.9% at the second follow-up. New cases of elevated TSH were observed in 9.2% of patients at the first follow-up and in 6.6% at the second visit. In liver function tests, AST/SGOT levels were raised in 4.2% of participants at the first follow-up and in 3.6% at the second follow-up. ALT/SGPT levels were elevated in 8.4% of participants at the first follow-up and in 4.8% at the second visit. Additionally, ALP levels were found to be elevated in 12.6% of participants at the first and in 7.0% at the second follow-up. For the kidney function test panel, patients were assessed regarding creatinine, urea, and uric acid levels. Creatinine levels were raised in 0.8% of patients at the first follow-up and in 1.1% at the second visit. Elevated urea levels were found in only 2.5% of patients at the first and 1.6% at the second follow-up. Similarly, high uric acid levels impacted just 1.9% of patients at the first follow-up and 1.4% at the second follow-up appointment.
A peripheral blood smear was conducted for patients at baseline, the first follow-up, and the second follow-up. Transformed lymphocytes and activated monocytes were observed in 1.1% of the participants at the first follow-up and 0.8% at the second appointment. An abnormal absolute neutrophil count was detected in the peripheral smears of 16.5% of patients at the first follow-up and 11.7% at the second. In turn, an abnormal absolute eosinophil count was noted in 7.9% of participants at the first follow-up and in 4.6% at the second. Electrocardiogram (ECG) results were abnormal in 2.2% of the participants at the first follow-up, with diagnoses including blocks (37.5%), bradycardia (25%), low voltage complexes (12.5%), Q waves (12.5%), and arrhythmias (12.5%). No ECG abnormalities were reported at the second follow-up visit. At the first follow-up, 4.06% of participants presented with abnormal chest X-ray findings, which were identified in the parenchymal (66.67%), pleural (26.67%), and mediastinal (6.66%) regions. At the second follow-up, 2.16% had abnormal findings, with involvement of the parenchymal (62.5%), pleural (12.5%), and mediastinal (25%) regions (Figure 2).
Discussion
Our study clarifies the potential long-term symptoms experienced by patients diagnosed with COVID-19. To our knowledge, this is one of the first community-based prospective cohort studies to evaluate post-COVID-19 syndrome in India. Within our participant cohort, the prevalence of post-COVID-19 syndrome was alarmingly high, with nearly two-thirds reporting 1 or more post-COVID symptoms. This finding aligns with research by Huang et al. [12] in China in 2020, where approximately two-thirds (68%) of participants reported at least 1 post-COVID-19 symptom. In contrast, a prospective cohort study by Selvakumar et al. [13] in 2023 in Norway found that nearly half (48.5%) of participants experienced post-COVID-19 syndrome, a lower rate than our findings. This discrepancy may be attributed to differences in the age profiles of the participants, which ranged from 12 to 25 years in the Norwegian study, as well as the prevalence of the B.1.1.7 (Alpha) variant of SARS-CoV-2 in that region in 2020. In contrast, our study coincided with the predominance of the Omicron variant of SARS-CoV-2. Another investigation by Naik et al. [10] in 2021 examined the clinical features and risk factors associated with post-COVID-19 signs and symptoms in a Northern Indian population. There, only 9.9% of participants reported symptoms 12 weeks following COVID-19 infection. The difference in findings might stem from the larger sample size of the latter study and the dominance of the SARS-CoV-2 Delta variant during their research period. Additional studies conducted around the world with similar objectives have reported a higher prevalence of post-COVID-19 syndrome (Table 1) [14,15].
In the present study, 25.2% of participants were diagnosed with 1 or more comorbidities at baseline, a figure that was 26.56% at the second follow-up visit. This finding is consistent with a study by Menges et al. [16] in 2021, which found that 34% of participants reported at least 1 chronic comorbidity at baseline. Our study indicated that 12.1% of participants had hypertension and 9.7% had diabetes mellitus. By comparison, a study by Cioboata et al. [17] in 2022 reported 36% of participants as exhibiting hypertension and 14.52% as having diabetes. The discrepancy may be attributable to the Alpha strain of COVID-19, which was present during the period of the latter study. We found no statistically significant relationship between the incidence of post-COVID-19 syndrome and the presence of any comorbidity. A 2023 study by Falsetti et al. [18] analyzed 3 years of data to assess whether the burden of comorbidities correlates with COVID-19 outcomes. Their nationwide analysis suggested that the development of post-COVID-19 symptoms was associated with a higher burden of comorbidities (p<0.0001). However, differences in sample size and study period, as well as the involvement of a different COVID-19 strain, could explain the variations in these findings. In our study, 93.5% of participants had received 2 doses of a COVID-19 vaccine at the time of baseline data collection. This aligns with national data from India, which indicated a vaccination coverage of 90% in 2022–2023 [19]. We observed no significant difference in post-COVID-19 symptoms between the vaccinated and unvaccinated cohorts (Table 1).
In the present study, we found no significant differences in liver function or kidney function parameters between the groups of patients with and without post-COVID-19 syndrome. The reported values were comparable between the groups. This is consistent with the findings of Alfadda et al. [20] in 2022, who investigated clinical and biochemical parameters in individuals 6 months post-recovery from COVID-19. Furthermore, we observed a decrease in lymphocyte count from baseline to the second follow-up in our study. Gameil et al. [21] in 2021 reported similar findings when assessing long-term biochemical residue after COVID-19 recovery. Additionally, we noted a decline in monocyte counts in participants at follow-up, which aligns with the findings of Ruenjaiman et al. [22] in their study on the response of innate immune cells after recovery from SARS-CoV-2 infection. At the 3-month follow-up, 4.06% of our participants presented with abnormal chest X-ray findings. This is notably lower than the 50.4% reported by Fogante et al. [23], who observed chest X-ray abnormalities in participants at the same follow-up interval. The discrepancy between these findings may be attributed to differences in methodology; specifically, Fogante et al. [23] focused on hospitalized patients, which could account for the higher rate of abnormalities. In our cohort, only 2.2% of participants exhibited ECG changes at the first follow-up, with no ECG changes observed at the second follow-up. Ovrebotten et al. [24] conducted a study assessing changes in cardiac structure and function 3 to 12 months after hospitalization for COVID-19 and found that, regardless of the severity of the initial illness and persistent dyspnea, cardiac structure and function remained unchanged.
A major strength of our study is the confirmation that all participants tested positive for SARS-CoV-2 via RT-PCR testing, thus minimizing the potential for misclassification. The data collection form employed in our study adhered to the standard format established by the WHO and underwent rigorous pretesting. The field staff responsible for conducting the interviews were well-trained and possessed a strong understanding of COVID-19. Although the sampling technique was robust in minimizing bias, its generalizability to larger populations may be limited by the small sample size.
On that note, our study has several limitations. First, the absence of baseline clinical profiles for the participants prior to COVID-19 infection represents a major drawback. Second, since participants self-reported their symptoms experienced after COVID-19, a potential exists for recall bias. Third, we were unable to determine whether the post-COVID-19 symptoms were attributable to COVID-19 vaccination, reinfection, or post-COVID-19 syndrome. Furthermore, while it would have been possible to include a variety of factors—such as data from the acute phase—in data collection from the patients’ medical histories, we limited the scope to the after-effects of COVID-19 infection to keep the study manageable. Finally, although we presume that the Omicron variant was predominant during the study period, we lack definitive evidence to confirm this.
Conclusion
In our study, the most common post-COVID-19 symptoms were joint pain, dry cough, anxiety, and shortness of breath. The most frequently reported comorbidities were obesity, hypertension, and diabetes. The longest median duration of symptoms was observed for loss of appetite, followed by anxiety and shortness of breath. Both sex and age were significantly associated with the presence of post-COVID-19 syndrome. Participants with any comorbidity in conjunction with post-COVID-19 syndrome exhibited a significantly longer duration of forgetfulness than participants without comorbid conditions. Additionally, we noted a general trend of significant improvement in ECG and chest X-ray findings at subsequent follow-ups.
The study findings suggest that clinical symptoms persist in participants for up to 6 months, with multi-system involvement observed during the post-COVID-19 period. Consequently, this necessitates long-term, regular follow-up appointments. As the post-COVID-19 sequelae are not well-defined, it is crucial to continue researching the long-term effects of COVID-19 to better understand how to treat and prevent post-COVID-19 syndrome. In conclusion, our study supports the use of a comprehensive healthcare approach to holistic patient management.
HIGHLIGHTS
In this study, out of 413 participants studied, 79.7% experienced post-coronavirus disease 2019 syndrome. In which, most frequently reported symptoms were fatigue (34.1%), bodyache (24.4%), dry cough (23.4%), and anxiety (20.9%). Loss of appetite persisted for the longest duration, with a median of 156.50 days, whereas reduced taste was the shortest-lived symptom (median 93 days). Forgetfulness lasted significantly longer amongst participants with comorbidities than those without comorbid conditions. Overall, biochemical parameters, peripheral smear results and electrocardiogram and chest X-ray findings indicated improvement at subsequent follow-up visits.
Supplementary Material
Supplementary data are available at https://doi.org/10.24171/j.phrp.2023.0251.
Distribution of study participants according to biochemical evaluations
Hematological and biochemical reference values
Notes
Ethics Approval
The Institutional Ethics Committee at MAMCand Associated Hospitals in New Delhi granted approval for the study, as documented in letter No. F.1/IEC/MAMC/87/05/2021/No526. All potential participants were informed about the study’s purpose, potential risks, and benefits. They were assured of confidentiality and informed that they had the option to withdraw from the study at any time if they wished. Written informed consent was obtained from all participants. For medical/telehealth consultations and follow-up appointments, participants were referred to LNH.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
The research received support from the Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Government of India. This support was provided in the form of funding through grant No. CTU/Cohort study/17/1025/2021/ECD. We express our gratitude for this assistance, which was instrumental in advancing our study and enhancing our comprehension of these key issues.
Availability of Data
The data supporting the findings of this study are available upon request from the corresponding author. They are not publicly accessible due to privacy or ethical restrictions.
Authors’ Contributions
Conceptualization: NB, MMS, SM, TA, SuS, SG; Data curation: HS, GS, US; Formal analysis: HS, SM, GS; Funding acquisition: NB, MMS; Investigation: HS, GS, SR, AB, TA, BG, SaS, MK, SuS, BK, NK; Methodology: NB, MMS, SM, TA, SuS, SG; Project administration: NB, MMS, HS, GS, SR, AB; Resources: NB; Software: HS, SM, GS; Supervision: NB, MMS, HS, SM, GS, SR, AB; Validation: NB, MMS; Writing–original draft: NB, MMS, HS, SM, GS; all authors. All authors read and approved the final manuscript.
Additional Information
This study was preprinted in medRxiv on August 25, 2023 (https://doi.org/10.1101/2023.08.25.23294654).
Acknowledgements
The authors would like to thank Dean, MAMC for providing support in the course of the conduction of the study. We also express thanks to the team of Doctors For You, Radiology Department LNH and NDTB, Delhi for providing necessary diagnostic support.