A Mycobacterium bovis outbreak among exhibition animals at a zoo in the Republic of Korea: the first contact investigation of zoonotic tuberculosis
Article information
Abstract
Objectives
Between July 2, 2021, and September 20, 2022, a Mycobacterium bovis outbreak occurred among exhibition animals at a zoo in the Republic of Korea. This study was conducted to assess the likelihood of M. bovis transmission to human contacts through a contact investigation and to implement preventive treatment for latent tuberculosis infection (LTBI).
Methods
In this descriptive study, the Korea Disease Control and Prevention Agency conducted a contact investigation, which included interviews, interferon-gamma release assay (IGRA) tests, and chest X-rays. Contacts underwent IGRA testing on 2 occasions: initial testing of 29 contacts (15 in the first cluster of infection and 14 in the second cluster) and follow-up testing of the 15 contacts in the first cluster.
Results
The study included 29 participants, 18 of whom were male (62.1%) and 11 female (37.9%). The mean participant age was 37.3 years (standard deviation, 9.6 years). In the initial IGRA tests, 6 of the 29 participants tested positive, indicating a prevalence of 20.7%. Following prolonged exposure, 1 additional positive case was detected in follow-up testing, raising the prevalence of LTBI to 24.1%. None of the contacts had active tuberculosis. Among the 7 individuals with positive results, 2 (28.6%) underwent treatment for LTBI.
Conclusion
This study faced challenges in confirming the transmission of M. bovis infection from infected animals to humans in the Republic of Korea. Nevertheless, adopting a One Health approach necessitates the implementation of surveillance systems and infection control protocols, particularly for occupational groups at high risk of exposure.
Introduction
Zoonotic tuberculosis (TB) is a form of TB that affects humans and is caused by Mycobacterium bovis, a member of the Mycobacterium tuberculosis complex (MTBC) [1–3]. M. bovis is responsible for the majority of TB cases in cattle and also infects a wide range of domestic and wild mammal species, leading to a chronic and progressive bacterial disease [4–6]. Transmission of M. bovis can occur among animals, from animals to humans, and from humans to animals, although human-to-human transmission is rare [7]. Humans can contract M. bovis through the inhalation of aerosols when in close contact with infected cattle or their carcasses. In addition, infection is associated with consuming unpasteurized dairy products or raw meat from infected cattle [8,9].
In 2020, the World Health Organization estimated that out of 10 million new cases of active TB, 140,000 (1.4%) were zoonotic TB, with approximately 11,400 (8.1%) resulting in death [10]. The United States Centers for Disease Control and Prevention reported in 2014 that M. bovis caused about 1.6% of all cases of TB in humans [11]. However, these global estimates are imprecise due to the lack of routine surveillance data from both human and animal populations [12]. In the Republic of Korea, no cases of M. bovis infection have yet been reported among Korean-born individuals [13,14]. The absence of effective surveillance programs hampers the accurate assessment of the disease’s burden. Moreover, the detection and identification of M. bovis cases in humans are particularly challenging given the current TB management systems for humans and livestock in the Republic of Korea. This difficulty arises because information and data are managed separately for different infected groups (humans, livestock, and exhibition animals), without an integrated approach.
From July 2, 2021, to September 20, 2022, a zoo in the Republic of Korea experienced an outbreak of M. bovis among its exhibition animals. In January 2021, out of 56 animals on display, 6 consecutive deaths occurred and were attributed to respiratory symptoms. Following these incidents, 43 of the remaining 50 animals tested positive for animal TB using polymerase chain reaction (PCR) testing. In response to the outbreak, the zoo’s health manager initiated an investigation into individuals who had been exposed to M. bovis and carried out TB screenings for both direct and indirect contacts. However, these examinations were not conducted in accordance with the protocol set forth in the Korean national guidelines for TB [15].
According to international research on risk factors for M. bovis infection in humans, such infection is closely associated with occupational exposure [8,16–18]. Transmission through occupational exposure can happen by inhaling aerosols exhaled by infected animals or humans [17]. It can also occur through direct contact, often in situations where wounds or injuries are present [17]. Despite a delay in sharing outbreak information, the Korea Disease Control and Prevention Agency (KDCA) opted to initiate contact investigations and preventive measures due to concerns about the possible spread of zoonotic disease.
Therefore, the objective of this study was to assess the likelihood of M. bovis transmission to human contacts through contact investigation and to implement preventive treatment for latent tuberculosis infection (LTBI). The findings may enhance our understanding of the occurrence and transmission mechanisms of zoonotic TB, with the ultimate goal of aiding in the development of prevention and control strategies for high-risk occupational groups.
Materials and Methods
Outbreak Recognition
From July 2, 2021, to September 20, 2022, an outbreak of M. bovis occurred among exhibition animals imported from South America to a zoo located in Gyeonggi Province, Republic of Korea [19]. These animals were housed in the zoo’s South American Pavilion, which is divided into 3 main sections: an outdoor exhibition space featuring llamas and guanacos, an open-air field where species such as giant anteaters and South American tapirs are kept, and an indoor exhibition hall connected to the open-air field. The zoo is home to approximately 370 species, 144 of which are endangered. As of January 2021, the pavilion showcased a total of 56 animals. Between January and May 2021, 6 animals—2 guanacos and 4 llamas—exhibited respiratory symptoms, including coughing and labored breathing, and subsequently died. In response, the zoo restricted access to the pavilion starting on June 5, 2021, and sought assistance from the National Institute of Wildlife Disease Control and Prevention (NIWDC), under the Ministry of Environment (ME), to perform PCR tests for TB on the 23 animals that had shared habitats with those that had died. On July 2, 2021, the first cluster of M. bovis infection was confirmed in 5 animals, consisting of 3 guanacos and 2 South American tapirs. Following the initial cluster, sporadic cases continued to emerge, leading to the confirmation of a second large-scale cluster on September 20, 2022. This cluster included 22 animals—6 llamas, 5 guanacos, 4 capybaras, 3 maras, 2 collared peccaries, and 2 South American tapirs—testing positive for M. bovis (Figure 1). As result of, from July 2, 2021, to September 20, 2022, 43 of the 50 animals exhibited in the pavilion were confirmed to be infected with M. bovis using PCR (Figure 2). During this period, 52 animals either died or were euthanized, which included 7 that were housed in the same enclosures as infected animals and 2 capybara cubs. None of the animals in the South American Pavilion survived.

Epidemic curve of a Mycobacterium bovis outbreak among exhibition animals at a zoo in the Republic of Korea (n=56).
NIWDC, National Wildlife Disease Control Center; TB, tuberculosis; PCR, polymerase chain reaction.

Distribution of animals with confirmed Mycobacterium bovis infection at a zoo in the Republic of Korea (n=43).
The zoo outbreak of M. bovis infection went undetected for a prolonged period. The KDCA identified the outbreak following notification from the Zoonosis Countermeasure Committee on December 23, 2022 [20]. In response, the KDCA organized a contact investigation following the protocols specified in Article 49 of the Infectious Disease Prevention and Control Act and Article 11 of the Tuberculosis Prevention Act [21,22].
Study Design and Study Population
This study is descriptive in nature, as we prospectively collected and analyzed data on contacts identified by the zoo’s health manager in both July 2021 and September 2022. Additionally, the study included only an exposure group, without a non-exposure group to serve as a control.
The total number of human contacts during the outbreak period was 36, with 15 contacts identified in July 2021 and 21 contacts in September 2022. Of these, 7 individuals were identified as contacts in both clusters. Consequently, our study population comprised 29 individuals, consisting of zookeepers, veterinarians, veterinary assistants, a laboratory technician, and maintenance staff.
Case Definition
The term “zoonotic tuberculosis” is commonly used in the medical literature to describe human TB disease resulting from infection with M. bovis [23]. However, according to the 2023 Case Definitions for National Notifiable Infectious Diseases published by the KDCA [24], TB represents an infectious disease caused by the MTBC, by the MTBC, the diagnosis of zoonotic TB the diagnosis of zoonotic TB caused by M. bovis is not specifically differentiated. Consequently, for the purposes of this study, we have defined zoonotic TB as a case in which a patient with TB has had M. bovis identified by genotype analysis conducted by the KDCA.
The term “outbreak,” in the context of TB, is not commonly defined in the literature due to the disease’s extended latency period [25]. Nevertheless, for the purposes of this study, we adopted this term to describe a large-scale incidence of M. bovis infection. Specifically, we defined an outbreak as the occurrence of 3 or more epidemiologically linked cases of confirmed M. bovis infection among exhibition animals between July 2021 and September 2022. This definition aligns with the guidelines for contact investigations issued by the US Centers for Disease Control and Prevention [25,26].
A “cluster” of TB cases is defined as 2 or more cases that share both epidemiological links and laboratory profiles, including genomic and drug susceptibility characteristics [25]. In the present study, culturing M. bovis was unsuccessful; instead, M. bovis infection was detected using PCR testing. To reduce variability in testing proficiency, methodology, and equipment, we categorized the cases into 2 clusters based on PCR results obtained from the NIWDC. Consequently, 2 distinct infection clusters were identified during the outbreak period: cluster #1 was recognized on July 2, 2021, and cluster #2 was detected on September 20, 2022.
This study entailed a secondary analysis of data derived from individuals identified as contacts. Evaluating the criteria used to select these contacts was not possible. Furthermore, no globally standardized definition or classification exists regarding “contact” with infected animals, which should consider variables such as the infectivity of the animals, the degree of exposure, and the proximity of contact. For the purposes of this study, “contact” was defined as an individual who had either direct or indirect contact with an infected animal, in accordance with the Korean national TB guidelines [15].
Data Collection
The zoo provided a list of 29 individuals along with the results of interferon-gamma release assays (IGRAs) and chest X-rays conducted in July 2021 and September 2022. In January 2023, we conducted a full contact investigation for these 29 individuals. Of these contacts, 8 had either resigned or been transferred. Therefore, contacts underwent IGRA tests and chest X-rays at local public health clinics either in the vicinity of the zoo or near their current residential addresses. We acquired the results of the chest X-rays and IGRA tests from these clinics and monitored the TB occurrence status among the contacts using the Integrated Tuberculosis Information System of the KDCA.
Contact Investigation
We carried out contact investigations in accordance with the Korean national TB guidelines, which included conducting interviews, IGRA testing, and chest X-rays [15]. Initially, we disseminated a survey form to local public health clinics to gather demographic information as well as epidemiological data, including occupation, department of work, exposure type, and exposure duration. Due to the extended duration of the outbreak and the risk of memory decay bias, we decided against collecting information on symptoms.
In July 2021, the zoo conducted IGRA tests and chest X-rays for the 15 contacts in cluster #1. For the 21 contacts in cluster #2, testing in September 2022 included only chest X-rays, with the tuberculin skin test (TST) and IGRA omitted. Consequently, in January 2023, a thorough contact investigation was initiated. This process included IGRA testing for all contacts from both clusters, for a total of 29 individuals.
LTBI testing of contacts
LTBI testing was conducted among contacts with normal chest radiograph findings to confirm their immune response to TB bacteria [27]. The current standard for diagnosing LTBI involves either TST or IGRA. In our study, we utilized IGRA due to its high specificity for LTBI diagnosis. This assay detects the cellular immune response to specific MTBC antigens, such as early secreted antigenic target-6 and culture filtrate protein-10, which are produced from genes in the RD1 region of the MTBC DNA [28,29].
In July 2021, the zoo initiated IGRA testing of 15 individuals from cluster #1, utilizing the QuantiFERON TB Gold Plus test (QFT-Plus; Qiagen). These contacts visited a private specialized diagnostic facility for blood sample collection. The test results were interpreted in accordance with the manufacturer’s instructions and subsequently communicated to each individual.
In January 2023, we carried out IGRA among 27 individuals. This included initial tests for 14 individuals in cluster #2 and follow-up tests for 13 individuals from cluster #1, excluding 2 who had tested positive initially. A total of 21 participants were tested using the STANDARD TB-Feron enzyme-linked immunosorbent assay (SD Biosensor), while the remaining 6 underwent testing with the QuantiFERON TB Gold Plus (QFT-Plus). The use of different test products stemmed from the varying practices of local public health clinics. The testing procedures were almost identical, with the primary difference being the use of 2 TB antigen tubes (TB1 and TB2) in the QuantiFERON TB Gold Plus kit [30]. Whole blood samples were collected in heparinized vacutainer tubes, following the sequence of nil tube, TB antigen tubes (including TB1 and TB2 for QFT-Plus), and mitogen tube. After thorough mixing, the tubes were transferred to the laboratory at the Gyeonggi Province Institute of Health and Environment for analysis. The results were interpreted using uniform criteria for determining positive, negative, and indeterminate outcomes, adhering to the guidelines provided by the manufacturers.
Clinical evaluation and testing for TB infection in contacts
The contacts were evaluated for symptoms and signs consistent with TB, and chest radiographs were performed to detect active pulmonary TB. When TB infection was suspected based on chest radiograph findings and respiratory symptoms, sputum microbiological tests, including sputum smear and culture, were scheduled.
Preventive treatment for LTBI and follow-up
The local public health clinics carried out monthly medical examinations and blood tests to identify potential side effects in contacts who had received preventive treatment. We tracked the incidence of TB among contacts with the aid of a digital TB surveillance system. In their duties as field workers, zoo staff members are required to participate in an annual national health examination that incorporates chest radiography to screen for active TB.
Data Analysis
The IGRA was administered to contacts on 2 separate occasions, with the results for each test presented individually. The prevalence of LTBI was determined by calculating the proportion of individuals who tested positive for IGRA within the study population. Conversion to positive IGRA was characterized by an initial negative IGRA result followed by a positive result upon follow-up.
The baseline characteristics of all enrolled contacts are presented using the mean and standard deviation for continuous variables, and frequencies and percentages for categorical variables. Descriptive statistics were used to compare the prevalence of LTBI among contacts according to sex, age, and occupation. Microsoft Excel ver. 2016 (Microsoft Corp.) was employed for the analysis.
Ethics Statement
This study was reviewed and approved by the institutional review board of the KDCA (No: KDCA-2023-04-10-PE-01) and conducted in accordance with the principles of the Declaration of Helsinki. It entailed a full contact investigation in accordance with the Tuberculosis Prevention Act and the Korean national guidelines for TB control, using data provided by the zoo. The requirement for informed consent was waived, as contacts signed separate consent forms for IGRA testing and LTBI treatment.
Results
Outbreak Investigation
The indoor area of the South American Pavilion, designed to mimic a tropical climate, is exceptionally hot and humid. The absence of adequate ventilation and humidity control in this space heightens the risk of TB infection. We hypothesized that the risk of transmission could affect not only the animals but also staff members who interacted with the infected animals and the contaminated environment. However, during the outbreak, staff members followed personal hygiene practices, such as wearing masks and practicing hand hygiene, due to the coronavirus disease 2019 pandemic. They also donned disposable personal protective equipment, including KF94 masks, protective clothing rated at Ingress Protection levels 5–6, nitrile gloves, and rubber safety shoes while performing autopsies. Consequently, the likelihood of transmission from the animals or the environment to the staff was deemed relatively low.
Contact Investigation
Characteristics of the study population
The study included 29 participants, 18 of whom were male (62.1%) and 11 female (37.9%). The mean age of the participants was 37.3 years, with a standard deviation of 9.6 years. We stratified their baseline characteristics by occupation. Zookeepers were the most numerous, with 10 individuals (34.5%), followed by 9 veterinarians (31.0%), 7 maintenance staff (24.1%), 2 veterinary assistants (6.9%), and 1 laboratory technician (3.4%). Zookeepers engaged in daily contact with animals for 1 hour while performing breeding tasks and assisted with specimen collection during the outbreak. Veterinarians were in contact with animals for about 1 hour weekly during medical procedures that involved collecting specimens, such as blood and nasal swabs. One veterinarian also had direct contact with animal tissues during autopsies. The maintenance staff were responsible for irregular duties, including the disposal of feces and sewage and the replacement of pipelines (Table 1).
IGRA
In the initial IGRA results, 6 of the 29 participants tested positive, yielding a prevalence of 20.7%. Subsequently, of the 13 individuals from cluster #1 with initial indeterminate or negative results, 1 person tested positive on follow-up due to prolonged exposure. Consequently, the prevalence of LTBI rose to 24.1% (7 of 29), as shown in Figure 3.

Investigation of contacts with animals infected with Mycobacterium bovis at a zoo in the Republic of Korea. Seven staff members in cluster #2 experienced dual exposure during the M. bovis outbreak period.
IGRA, interferon-gamma release assay; LTBI, latent tuberculosis infection.
Initial IGRA
The initial IGRA was administered to a total of 29 individuals. In cluster #1, the 15 contacts were tested at the time of exposure. The 14 contacts in cluster #2 underwent the test 24 weeks following their last exposure.
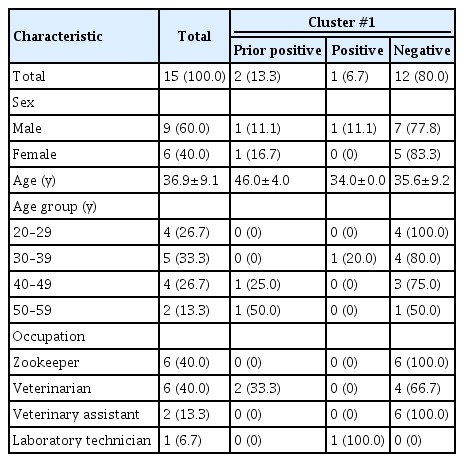
Among the 15 contacts in cluster #1, 2 individuals tested positive, representing an LTBI prevalence of 13.3%. The sex distribution of the positive cases was evenly split, with 1 male and 1 female contact,The sex distribution of the positive cases was evenly split, with 1 male and 1 female contact. The positive cases had a higher mean age, at 46.0±4.0 years, compared to 35.6±9.2 years for the negative cases. Both of the individuals with positive results were veterinarians, which corresponds to a 33.3% prevalence of LTBI within this occupational group.
Among the 14 contacts in cluster #2, 4 tested positive, resulting in a 28.6% prevalence of LTBI. Regarding sex distribution, LTBI was diagnosed in 3 male and 1 female. The mean age of the contacts was 37.7±10.1 years, with no meaningful difference between the IGRA-positive and IGRA-negative groups. When examining LTBI prevalence by occupation, veterinarians had the highest proportion at 33.3%, followed by maintenance staff at 28.6% and zookeepers at 25.0%. One veterinarian reported a family history of TB, but this individual fortunately received a negative IGRA result.
In summary, of the 29 individuals who underwent initial IGRA testing, 6 tested positive, indicating a 20.7% prevalence of LTBI. cluster #2 exhibited a higher prevalence of IGRA positivity at 28.6%, compared to 13.3% in cluster #1. Notably, the mean age of IGRA-positive individuals in cluster #1 was higher (46.0±4.0 years) than in cluster #2 (37.3±9.7 years). Additionally, veterinarians constituted the largest proportion (33.3%) of IGRA-positive individuals in both clusters, as shown in Table 2.
Follow-up IGRA
The zoo did not perform follow-up testing 8–10 weeks after the initial IGRA for contacts in cluster #1 who initially tested negative. However, the KDCA opted to conduct follow-up IGRA for these contacts, despite 72 weeks having elapsed since the initial test. Of the 15 contacts, 1 individual was newly diagnosed with LTBI, in addition to the 2 who had tested positive initially. The new positive case is a 34-year-old laboratory technician who had received an indeterminate result on the initial test. Therefore, among the 7 individuals with prolonged exposure during the outbreak, 3 tested positive either during the initial testing or follow-up testing (Table 3).
Clinical evaluation and chest radiograph
Contacts were physically examined to detect signs and symptoms of active TB; however, none displayed evidence of the disease. All chest radiographs showed normal findings.
Preventive treatment and follow-up
Among the 7 contacts diagnosed with LTBI, 2 (28.6%) consented to undergo treatment, while 5 (71.4%) declined. The prescribed LTBI treatment regimen consisted of a 3-month course of combined isoniazid and rifampin. Both individuals who commenced treatment completed it successfully, without experiencing adverse effects. As of September 30, 2023, no active TB cases among the 29 contacts had been reported in the KDCA’s digital TB surveillance system. In preparation for potential TB infections among contacts, the NIWDC is preserving tissue samples from animals with confirmed M. bovis infection to facilitate epidemiological linkage by genetic analysis.
Discussion
The KDCA conducted a comprehensive investigation into the M. bovis outbreak among exhibition animals that extended from July 2, 2021, to September 20, 2022. They also performed IGRA testing and chest X-rays for zoo staff at risk of M. bovis transmission.
In this study, the prevalence of LTBI among contacts was 20.7% (6 of 29) in initial testing and 24.1% (7 of 29) in follow-up evaluation. A thorough review of the international literature revealed several factors that complicated a comparison of our research results with data from other countries. Zoonotic TB is strongly associated with economic and sociocultural factors, such as the consumption of unpasteurized dairy products and the sharing of living spaces with animals [11]. However, in the Republic of Korea, the sale of raw milk is prohibited by the Livestock Products Sanitary Control Act (Articles 4 and 32) [31], and the living spaces of livestock and humans are strictly separated. In the present study, LTBI testing was conducted to prevent secondary cases of TB. The mean age of our study population was 37.3±9.6 years. Therefore, we compared our findings with the 35–49-year-old subgroup within group A (representing workers at postpartum care centers, social welfare institutions and educational facilities) from a large-scale LTBI study using IGRA, conducted by the government of the Republic of Korea in 2017–2018 [32]. Despite the limited number of contacts, the prevalence of LTBI in our study resembles that of the general population (19.9%) reported in the reference literature. Prior research indicates that as individuals age, their pulmonary immune responses deteriorate, contributing to an increased susceptibility to TB [33]. Age has been identified as the variable most strongly influencing the prevalence of LTBI, which increases with advancing years [32]. However, in our study, the largest number of LTBI cases (3 individuals) was found in the 40–49-year age group, followed by 2 individuals in the 30–39-year age group and 1 individual each in the 20–29-year and 50–59-year groups. Age appeared to exhibit no direct correlation with the incidence rate of LTBI. Regarding the distribution of IGRA positivity by occupation, 3 of the 7 contacts with positive results were veterinarians, followed by 2 maintenance staff members, 1 zookeeper, and 1 laboratory technician. Unfortunately, only 2 individuals (28.6%) underwent treatment for LTBI, as the veterinarians and a laboratory technician in the high-risk exposure group refused treatment due to their asymptomatic status and considerations related to pregnancy.
Zoonotic TB is clinically and pathologically indistinguishable from TB caused by M. tuberculosis [22]. This disease requires careful management because M. bovis is inherently resistant to pyrazinamide, a crucial first-line medication [1], and may be more deadly than M. tuberculosis, as it more frequently results in miliary and central nervous system TB [3]. Differentiating among the members of the MTBC is essential for epidemiological studies in human cases and for the appropriate treatment of patients with TB. However, the genetic sequence homogeneity within the MTBC exceeds 99.9%, which poses challenges in distinguishing between individual strains [34]. International studies suggest that the likelihood of M. bovis transmission from animals to humans is low [21]. However, the risk of infection is higher among certain occupational groups [16–18]. Notably, the occupational risk for M. bovis exposure is greater for individuals working in enclosed spaces with close contact during animal examination, treatment, or handling, which increases the potential for transmission [17,35]. As a preventive measure, workers should consistently wear personal protective equipment, and workspaces should enforce proper ventilation and infection control measures. Additionally, high-risk occupational groups, including veterinarians performing necropsies, abattoir workers, livestock farmers, and dairy farmers, should regularly monitor their health status [36]. Other occupational groups at risk of contracting M. bovis from animals include laboratory workers, as well as those employed in wildlife parks, zoos, and animal houses [36]. However, following the implementation of the Tuberculosis Prevention Act in the Republic of Korea [21], zoo staff members have not been subjected to mandatory LTBI examinations. Prior to the M. bovis outbreak at the zoo, these workers had not undergone TST or IGRA testing. Furthermore, contact with contaminated water or soil represents a meaningful route of transmission, as M. bovis can survive in the environment for extended periods after being expelled in aerosols [20,37,38]. To eliminate the source of infection, early detection of preclinical M. bovis infection in animals and the prompt removal of all infected animals are necessary [39,40].
In the Republic of Korea, the Animal Plant Quarantine Agency (APQA) is working to eliminate TB from cattle through a “test-and-slaughter” strategy [41]. These preventive measures have contributed to a gradual decrease in animal TB cases. However, demand is rising for more sophisticated preventive policies, such as vaccination, to ensure animal health. ME has introduced the First Comprehensive Plan for Zoo Management (2021–2025) to improve zoo management practices [42]. Despite these efforts, no legal framework currently governs screening for zoonotic infectious diseases, including TB, when animals are imported for exhibition or introduced to new facilities. In terms of human health, local public health clinics evaluate individuals who have been in contact with cattle diagnosed with LTBI using the TST or IGRA. These clinics perform chest radiographs to check for TB but do not conduct LTBI examinations, and they report their findings to the KDCA [14]. However, sometimes inspections and reporting fail to occur due to ineffective information sharing between regional human and animal health departments. The situation described in this report underscores the importance of recognizing TB as a zoonotic disease and provides a direct impetus for promoting intersectoral cooperation among the human, animal, and environmental health sectors in the Republic of Korea. To this end, the tripartite organizations (the KDCA, APQA, and ME) launched the Joint Epidemiological Investigation Manual for Zoonotic TB on May 31, 2023, to enhance collaborative responses across relevant sectors [43]. A key meeting took place on June 27, 2023, to improve coordination and communication between national and regional authorities responsible for human and animal health. The KDCA has also established a new act to facilitate the collection of digital data, which will be linked to the Integrated Tuberculosis Information System and the Korea Animal Health Integrated System [44]. The KDCA’s Third National Strategic Plan for Tuberculosis Control (2023–2027) includes the implementation of a TB pathogen genotyping surveillance system, targeting high-risk occupational groups such as veterinarians and zookeepers [45]. Additionally, on June 20, 2023, the organization updated its case report forms to screen occupational risk groups among patients with TB [46].
This study had several limitations. First, we could not determine whether individuals who tested positive for IGRA were infected with M. bovis, since IGRA returns a positive result for TB bacterial antigens within the MTBC. Second, due to the small sample size, we cannot rule out the possibility of underestimation or overestimation of the IGRA results. Additionally, the small sample size precluded the performance of statistical significance testing of the IGRA results by variable. Third, given the prolonged incubation period of TB, it is difficult to definitively exclude the chance that individuals who tested positive for IGRA may have acquired the infection before the outbreak. Moreover, as the testing interval increases, so does the likelihood of exposure to various sources, which complicates the generalization of IGRA test results. Therefore, we recommend collecting additional data from high-risk occupational groups to identify cases of TB and LTBI and to conduct a risk factor analysis for M. bovis infection.
Conclusion
To our knowledge, this is the first report to document a contact investigation and the use of IGRA to test human contacts of exhibition animals with confirmed M. bovis infection. In this Korean study, confirming transmission of M. bovis from infected animals to humans was challenging due to the limited number of contacts and a prevalence of LTBI resembling that of the general population. Nonetheless, within the framework of a One Health approach, a clear need exists for surveillance systems and infection control protocols tailored to groups at high risk of occupational exposure. The insights gained from this study are anticipated to inform the development of improved policies and prevention strategies for zoonotic TB.
HIGHLIGHTS
• A Mycobacterium bovis outbreak occurred among exhibition animals at a zoo in the Republic of Korea from July 2, 2021, to September 20, 2022.
• The initial prevalence of latent tuberculosis infection (LTBI) was 20.7% (6 of 29 contacts), which increased to 24.1% (7 of 29) after follow-up testing.
• The 7 individuals with positive interferon-gamma release assays included 3 veterinarians, 2 maintenance staff, 1 zookeeper, and 1 laboratory technician. Of these, 2 individuals (28.6%) underwent treatment for LTBI.
• This study increases awareness of zoonotic tuberculosis, underscoring the necessity for a tuberculosis surveillance system targeting high-risk occupational groups within the One Health framework.
Notes
Ethics Approval
This study was reviewed and approved by the institutional review board of the KDCA (No: KDCA-2023-04-10-PE-01) and conducted in accordance with the principles of the Declaration of Helsinki. It entailed a full contact investigation in compliance with the Tuberculosis Prevention Act and the Korean national guidelines for tuberculosis control, using data provided by the zoo. The requirement for informed consent was waived, as contacts signed separate consent forms for IGRA testing and LTBI treatment.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
This study was supported by the KDCA of the Republic of Korea (Grant No: 6136-303-210-01).
Availability of Data
All data generated or analyzed during this study are included in the published article. Additional data can be requested from the corresponding author.
Authors’ Contributions
Conceptualization: HYL, YK; Data curation: HYL, YK; Formal analysis: HYL; Investigation: HYL, YK, JK; Methodology: HYL, YK, SEL, JK; Project administration: YK, SEL, HC; Software: HYL; Visualization: HYL; Writing–original draft: HYL; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Acknowledgements
We extend our deepest gratitude to the zoo staff and the officials at the local public health clinics for their invaluable cooperation with the contact investigation.