Factors that Correlate with Poor Glycemic Control in Type 2 Diabetes Mellitus Patients with Complications
Article information
Abstract
Objectives
Inadequate glycemic control amongst patients with Type 2 diabetes mellitus (T2DM) indicates a major public health problem and a significant risk factor for the progression and complications caused by diabetes. Glycemic control is the main therapeutic objective for the prevention of organ damage and other complications arising from diabetes.
Methods
This was a retrospective observational study of T2DM patients with complications, who were aged 40 years and older. The study was conducted retrospectively on medical records (in-patient and out-patient) obtained from a South Indian teaching hospital, Manipal, India. The patients included in the study had fasting blood sugar, postprandial blood sugar and HbA1c measured at least twice during follow-ups the previous year. Patients’ HbA1c levels were categorized into good control ≤7% (≤53mmol/mol), and poor control >7% (>53mmol/mol), and patients’ characteristics were analyzed.
Results
A total of 657 patients were included in the study. The mean age was 59.67 (SD = 9.617) years, with 152 (23.1%) females and 505 (76.9%) males, and 514 (78.2%) patients had poor glycemic control. Most of the patients were on insulin mono-therapy [n = 271 (42.1%)], about a third of the patients were on combination therapy that included an oral hypoglycemic agent and insulin [n = 236 (36.6%)]. Patients with a history of more than 10 years of diabetes [n = 293 (44.6%)], had a family history of diabetes [n = 256 (39%)] and obesity [n = 95 (14.5%)], all had poor glycemic control.
Conclusion
This present study indicated a significant association of gender (female), age, high-density lipoprotein level, duration of diabetes and type of medication, with poor glycemic control in T2DM patients that had secondary medical complications.
Introduction
Diabetes is a chronic condition caused by either an absolute lack of insulin or a relative lack of insulin due to impaired insulin secretion and action [1,2]. Insulin resistance and glucose intolerance results in hyperglycemia and alterations in lipid and protein metabolism [3]. In the long term, these metabolic abnormalities contribute to complications such as cardiovascular disease, retinopathy, nephropathy, and neuropathy [4–6]. Diabetes mellitus (DM) is very common in all age groups, worldwide [7–9]. The number of people with diabetes worldwide was estimated as 415 million in 2015, and is expected to rise to 642 million by 2040 [10].
There are several risk factors for the progression of Type 2 DM (T2DM) including family history, obesity, chronic physical inactivity, race or ethnicity, history of impaired fasting glucose, impaired glucose tolerance, HbA1c 5.7% to 6.4% (38.8mmol/mol to 46.4mmol/mol), hypertension, abnormal high-density lipoprotein cholesterol and/or elevated triglyceride levels [11]. The duration of diabetes, lifestyle, level of education, age, number of medications, morbidity, socioeconomic factors and type of insurance coverage, are risk factors for sustained poor glycemic control. Individuals at risk of poor glycemic control may need specific interventions to achieve optimal glycemic control [12].
Inadequate glycemic control among patients with T2DM indicates a major public health issue and a significant risk factor for the progression of diabetic complications. Glycemic control remains the main therapeutic target for prevention of organ damage and other complications arising from diabetes [13]. In clinical practice, achieving optimal glycemic control on a long-term basis is challenging, since the reasons for poor glycemic control in T2DM are complex [14]. Both patient and health care provider-related factors may play a significant role in poor glycemic control [15,16].
The glycosylated hemoglobin, or A1c has become the gold standard for measuring chronic glycaemia and is the clinical marker for predicting long-term complications, particularly microvascular complications [17–19]. HbA1c is most commonly measured because it comprises of the majority of glycosylated hemoglobin and is the least affected by recent fluctuations in blood glucose. In epidemiological analyses, glycated hemoglobin (A1c) levels >7% (>53mmol/mol) are associated with a significantly enhanced risk of both macrovascular and microvascular complications, irrespective of the main treatment [20–22]. People with diabetes have a greater risk of developing a number of major health problems. The costs related to diabetes include increased use of health services, disability and productivity loss, which can be a considerable burden to the patient, families and society.
T2DM is approaching epidemic levels in India [23]. The level of morbidity and mortality due to diabetes and its possible complications, are enormous and cause significant healthcare problems for both the family and society. Diabetes is associated with a variety of complications and is occurring at a relatively younger age in India [24]. In addition to directly related medical complications, numerous factors contribute to the impact of diabetes on quality of life, morbidity and early death in these patients.
The present study evaluated the factors which predict poor glycemic control as measured by glycosylated hemoglobin. Identifying predictors that contribute to poor glycemic control may enable future therapeutic modification or control of these factors for the management of T2DM.
Materials and Methods
This retrospective observational study was conducted based on in-patient and out-patient medical records of patients of Kasturba Hospital, Manipal, India. Medical records of patients who were admitted to the hospital during the 2-year time period (from August 2013 to September 2015) who were ≥ 40 years old, diagnosed with T2DM with complications, had fasting blood sugar, post-prandial blood sugar and HbA1c measured at least twice during the previous year, were included in the study.
The study was carried out according to the protocol approved by the Institutional Ethics Committee (IEC: 561/2015). Based on the study criteria and screening of 2,054 patient files, 657 patients who met the study criteria were included in the study.
Every reported visit of the patient to the hospital was followed, and patients’ clinical details were checked until the last visit of the patient. Demographic details like age, sex, occupation, body mass index (BMI), social habits, date of diagnosis of T2DM, number of hospitalizations and clinical parameters, medical and medication history, reports of laboratory investigations, and treatment charts, were all collected and documented in a case report form. For each patient, the mean of the previous two HbA1c levels was calculated and the patients were divided into 2 groups according to the mean HbA1c level, either good control group (HbA1c ≤7% or ≤53mmol/mol) or poor control group (HbA1c >7% or >53mmol/mol). Statistical analyses were carried out using SPSS Ver.20 and p ≤ 0.05 was considered statistically significant. Mean ± SD were used to summarize continuous variables and frequency, and percentage was used to summarize categorical variables. Chi-square test was used to examine the association between categorical variables. The binary logistic regression (univariate and multivariate) model was developed to test the predictors of poor glycemic control. ROC curve was used to check the classification ability of the model.
Results
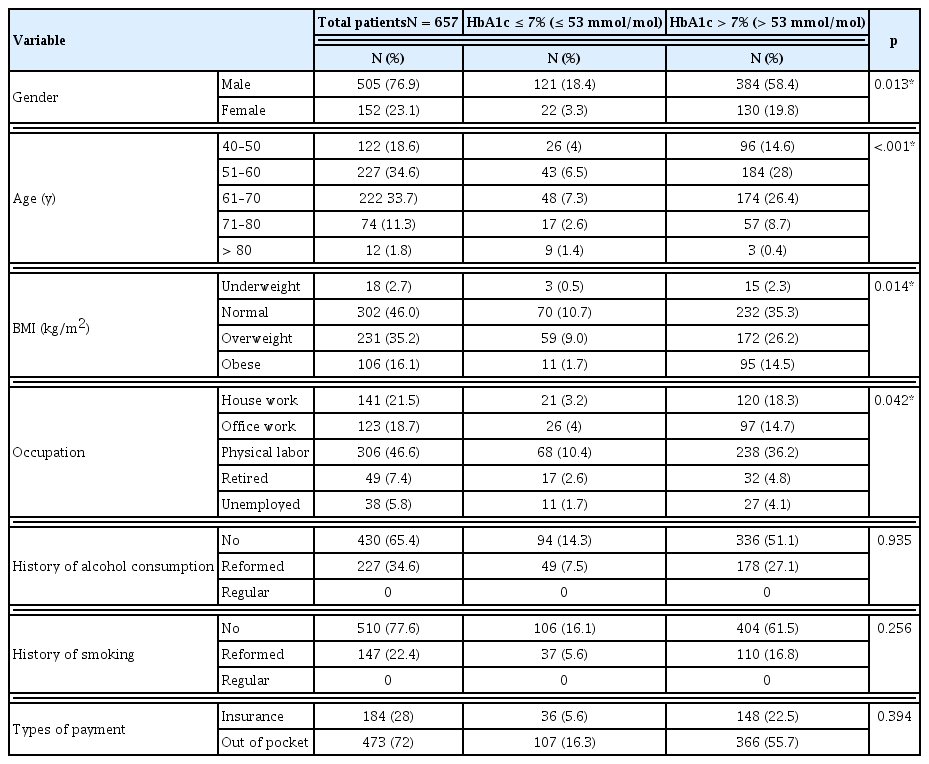
Out of 657 patients included in this study the mean age was 59.67 (SD = 9.617) years, and 505 (76.9%) were male, and the majority of all study patients were aged 51–70 years [n = 449 (68.3%)]. Most of the patients had a normal weight [n = 302 (46%)], 106 (16.1%) patients were obese (Table 1). Patients were suffering from different types of diabetic complications. Out of 657 patients, 514 (78.2%) had 1 diabetic complication and 143 (21.8%) had 2 complications. The majority of patients [n = 175 (26.6%)] were suffering from diabetic peripheral neuropathy, of which 148 (22.5%) were male, and 27 (4.1%) were female patients. Patients with diabetic retinopathy accounting for 109 (16.6%) males and 48 (7.3%) females. There were 306 (46.6%) patients suffering from cardiovascular disorders, including hypertension and dyslipidemia. In this study, 86 (13.1%) had infectious diseases, which were more common and serious in patients with T2DM. Patients without co-morbidity accounted for 182 (27.7%) patients.
Based on the nature of the patient’s job and physical activity, the study was divided into 5 categories. Most of the patients were physical laborers and houseworker, 46.6% and 21.5% respectively. The remaining were office workers (18.7%) retired (7.5%), or unemployed (5.8%). The majority of the study patients were non-alcoholics [n = 430 (65.4%)] and non-smokers [n = 510 (77.6%)], and 473 (72%) of the patients paid for their own medical care expenses (Table 1).
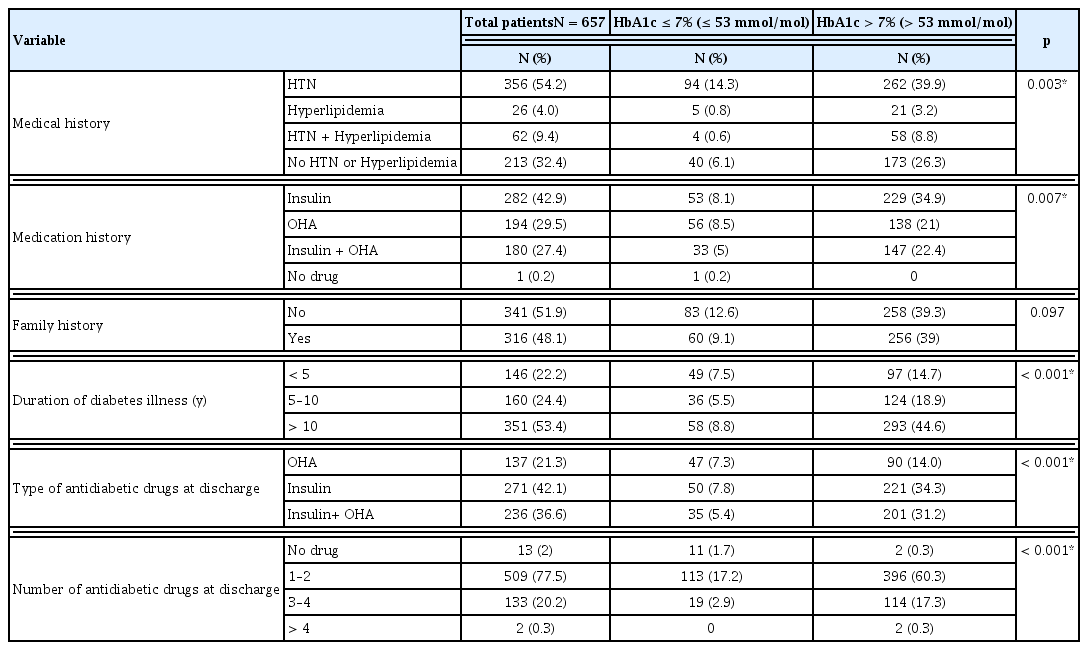
Over half the study patients 356 (54.2%) had a history of hypertension, 26 (4.0%) had hyperlipidemia, Patients that did not have any history of hypertension or hyperlipidemia accounted for 32.4%. There were 280 (42.6%) patients that used insulin to manage diabetes and 182 (27.7%) had a history of using combination therapy (insulin and oral hypoglycemic drug), 194 (29.5%) had used only oral hypoglycemic agents. One patient that was newly diagnosed for T2DM with complications, and the majority of the patients in the study did not have a history of diabetes in their family. Most of the patients [n = 351 (53.4%)] had been diagnosed with T2DM for more than 10 years. The remaining patients had T2DM for 5–10 years [n = 160 (24.4%)], and 146 (22.2%) had T2DM for less than 5 years (Table 2). Assessment of the drugs prescribed showed that 13 (2%) patients were not prescribed anti-diabetic medication. A combination of insulin and oral hypoglycemic agents were prescribed for 236 (36%) patients to manage their condition. Mostly, patients used insulin to control their blood glucose level [n = 271 (41%)], or oral anti-diabetics as monotherapy [n = 137 (21%)] (Table 2).
There was a significant association between HbA1c levels and demographic factors: gender, age, BMI and occupation. Most of the patients had a HbA1c level >7 % (>53mmol/mol) which represents poor glycemic control in these patients (Table 1). In patients with poor glycemic control, 262 (39.9%) had a history of hypertension, and 147 (22.4%) had a history of insulin and oral anti-diabetics drug prescription. In this study patients either with or without family history of diabetes, had poor glycemic control. There was a significant association between the duration of diabetes and HbA1c levels; 293 (44.6%) patients with poor glycemic control had been suffering from diabetes for more than 10 years. Patients that used insulin alone to control the glucose level accounted for 221 (34.3%) patients, 201 (31.2%) had combination therapy (OHA and insulin), and 396 (60.3%) had 1 or 2 forms of diabetes medication (Table 2).
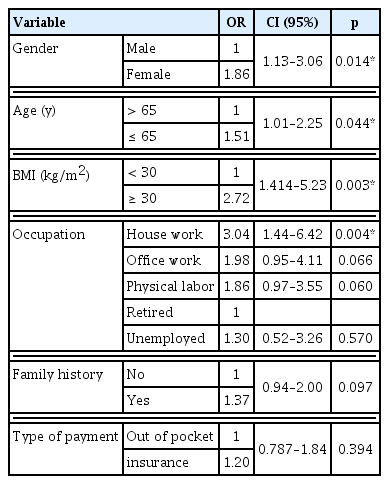
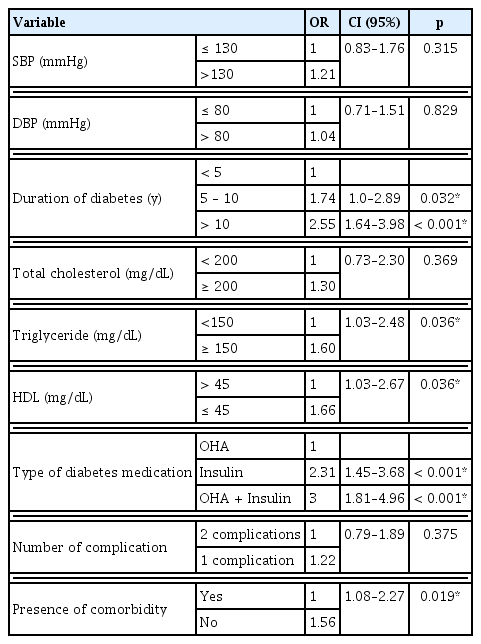
The risk of poor glycemic control was higher amongst females (OR = 1.86) and patients that were 65 years old or younger, (OR = 1.51) and who were obese (OR = 2.72). House wives were at a higher risk when compared to retired patients (OR = 3.04). Patients with family history were more likely to have poor control [OR = 1.37 (Table 3)]. Patients with a systolic blood pressure greater than 130mmHg were more likely to have poor glycemic control (OR =1.21), patients with a diastolic blood pressure greater than 80mmHg were also more likely to have poor glycemic control (OR = 1.04).
The longer a patient had diabetes the worse the glycemic control; 5 to 10 years duration (OR = 1.74), and in patients with a history of diabetes for more than 10 years compared to those with less than 5 years of illnesss (OR = 2.55). Patients without co-morbidity had significantly better glycemic control compared to patients with co-morbidity (OR=1.56). Other factors like total cholesterol, triglyceride level and the type of diabetes medications, all significantly affected glycemic control (Table 4).
The results of multivariate analysis showed that females (OR = 2.07), patients younger than 65 years old (OR = 1.67), abnormal high-density lipoprotein (HDL) level (OR = 1.72), duration of diabetes (more than 10 years), and type of diabetes medication, were all significantly associated with poor glycemic control (Table 5). The developed logistic regression model included significant variables that are associated with poor glycemic control (HbA1c as reference line). The developed model had an area under ROC curve of 0.683 (p < 0.001).
Discussion
Diabetes increases the risk of developing a number of major health problems. The level of morbidity and mortality due to diabetes, and its possible long-term complications can cause significant healthcare problems for both the family, and society [25]. Many factors can influence optimal glycemic control: gender, age, BMI, duration of illness, type of medication, lipid profile and blood pressure [26,27]. In this study, HbA1c value was used because it is the gold standard test for glycemic control. In diabetes patients good glycemic control is defined as having values of HbA1c ≤ 7% and poor glycemic control has (HbA1c values of >7% [28–30]. A total of 657 patients were included in this study; the majority of the patients had poor glycemic control (78.2%), males were predominant in this study, and a significantly higher risk of poor glycemic control was associated with females (p < 0.001). Roy et al [31] showed escribed sub-optimal control in males.
In this study, a significant association was found between glycemic control and age. Most patients with poor glycemic control belonged to the age categories 50–60 years and 60–70 years, which was similar to the studies reported by Huang et al [32] and Woldu et al [33]. This study observed a significant relationship between glycemic control in diabetic people and BMI (p = 0.014) and occupation (p = 0.042), similar studies by Lee et al [34] and Kassahun et al [35], who reported the effect of being overweight or obese, and occupation in T2DM.
History of hypertension or hyperlipidemia (p = 0.003) and the length of time a person has been diabetic (p < 0.001) were the other factors that were observed in this study to have a significant association with non-glycemic control. Other studies by Khattab et al [36] and Salonen et al [37] reported that a longer duration of diabetes, and both hypertension and dyslipidemia were associated with insulin metabolism disturbance and poor glycemic control. By studying the patients’ medication history and medications prescribed at discharge, a significant association between glycemic control and type of medication history (p = 0.007) was observed. Diabetes medication and the number of diabetic drugs in prescription at discharge was also significantly associated with glycemic control (p < 0.001). This finding is consistent with other studies carried out by Roy et al [31], Agarwal et al [38], Esposito et al [39] and Schweizer et al [40].
In this study, we did not find any statistically significant effects of factors like history of alcohol consumption or smoking, family history and type of medical expenses coverage, with glycemic control. According to another study by Juarez et al [12], the type of insurance coverage did not impact glycemic control significantly. The present study showed that male patients had better glycemic control and the risk of poor glycemic control was significantly higher amongst females and especially in women who are responsible for providing care to the family who may neglect their health care as reported by Kirk et al [41] and Zhao et al [42], the same results were found in this study. It has been observed that patients younger than 65 years old were significantly more likely to have poor glycemic control. Studies by Harrabi et al [43] and Eid et al [44] revealed that age has a significant effect on glycemic control. In a study by Adham et al [45], BMI was reported to impact on HbA1c level. In this study, the significant effects of obesity on poor glycemic control could be explained by impaired insulin resistance and insulin secretion. Another investigation reported by Bays et al [46], confirmed the association of being overweight or obese increase risk of developing diabetes. This study revealed that retired patients had significantly better glucose control compared to house wives and other categories of people. This could have been because retired people have enough time to manage their therapy and change their lifestyle. A survey by Kassahun et al [35], reported that poor glycemic control appeared to be greater amongst farmers compared to unemployed respondents. In the present study, patients who made self-payment for medical expenses appeared to be more likely to have better glycemic control compared to patients with insurance coverage, although this effect was not significant. This is in contrast to the results of a study by Juarez et al [12], where they reported that insurance coverage was not significantly related to glycemic control.
As reported by Papazafiropoulou et al [47] and Bo et al [48], no influence of family history on the clinical characteristics of patients with diabetes was found except for low-density lipoprotein cholesterol levels. In this study, it was observed that patients with a family history of diabetes were more likely to have poor glycemic control, but this effect was not statistically significant. In a study by Khattab et al [36] and Eid et al [44], it has been reported that the duration of T2DM was strongly associated with poor glycemic control. This study revealed similar results, a longer duration of diabetes adversely affected glycemic control, possibly due to a reduction in insulin secretion or excessive insulin resistance in those patients. In addition, a survey reported by Juarez et al [12] showed patients with diabetes for 6 to 7 years, or for 10 years or more were more likely to have wide glycemic variability compared to patients that had diabetes for 3 years or less. A longer duration of diabetes is the risk factor for sustained, poor glycemic control [12].
Lipid abnormalities are common in patients with diabetes. In this study, dyslipidemia was associated with poor glycemic control, especially for higher triglycerides ≥ 150 mg/dL. Studies by Adham et al [45] and Benoit et al [49] revealed that factors related to better glycemic control were lower levels of total cholesterol, low-density lipoprotein cholesterol and triglycerides. In this study, we found that the type of medication was significantly related to the level of HbA1c, in patients receiving insulin + OHA or insulin as mono-therapy were more likely to have poor glycemic control compared to patients who were on oral diabetes medication. This could be due to implementation of an insulin regimen or having an optimal glycemic level that could not be achieved by oral medication alone. The finding is consistent with other reported studies by Khattab et al [36] and Benoit et al [49]. As indicated by El-Kebbi et al [50], co-morbidity does not appear to limit achievement of good glycemic control in patients with T2DM. Patients with more than 1 complication of diabetes appeared to have had better glycemic control compared to patients who were suffering from 1 complication but was not statistically significant. Multivariate analysis indicated a significant association of gender (female), age, HDL level, duration of diabetes illness and type of medication, with poor glycemic control.
Conclusion
The present study showed that there was a significant association between certain demographic factors like gender, age, BMI, occupation and clinical variables like medical history, medication history, triglyceride level, HDL level, duration of diabetes illness, type and number of prescribed diabetes medication, with HbA1c level. Based on these factors, patients at risk of poor glycemic control can be identified, and targeted interventions can be implemented for optimal outcomes. Factors such as level of adherence, physical activity, diabetes education and training programs also impact on the optimal glycemic control, although these factors were not analyzed in this study.
Notes
Conflicts of Interest
The authors declare that there was no conflict of interest associated with this paper.