Abstract
-
Objectives
- On November 5, 2021, Pfizer Inc. announced Paxlovid (nirmatrelvir+ritonavir) as a treatment method that could reduce the risk of hospitalization or death for patients with confirmed coronavirus disease 2019 (COVID-19).
-
Methods
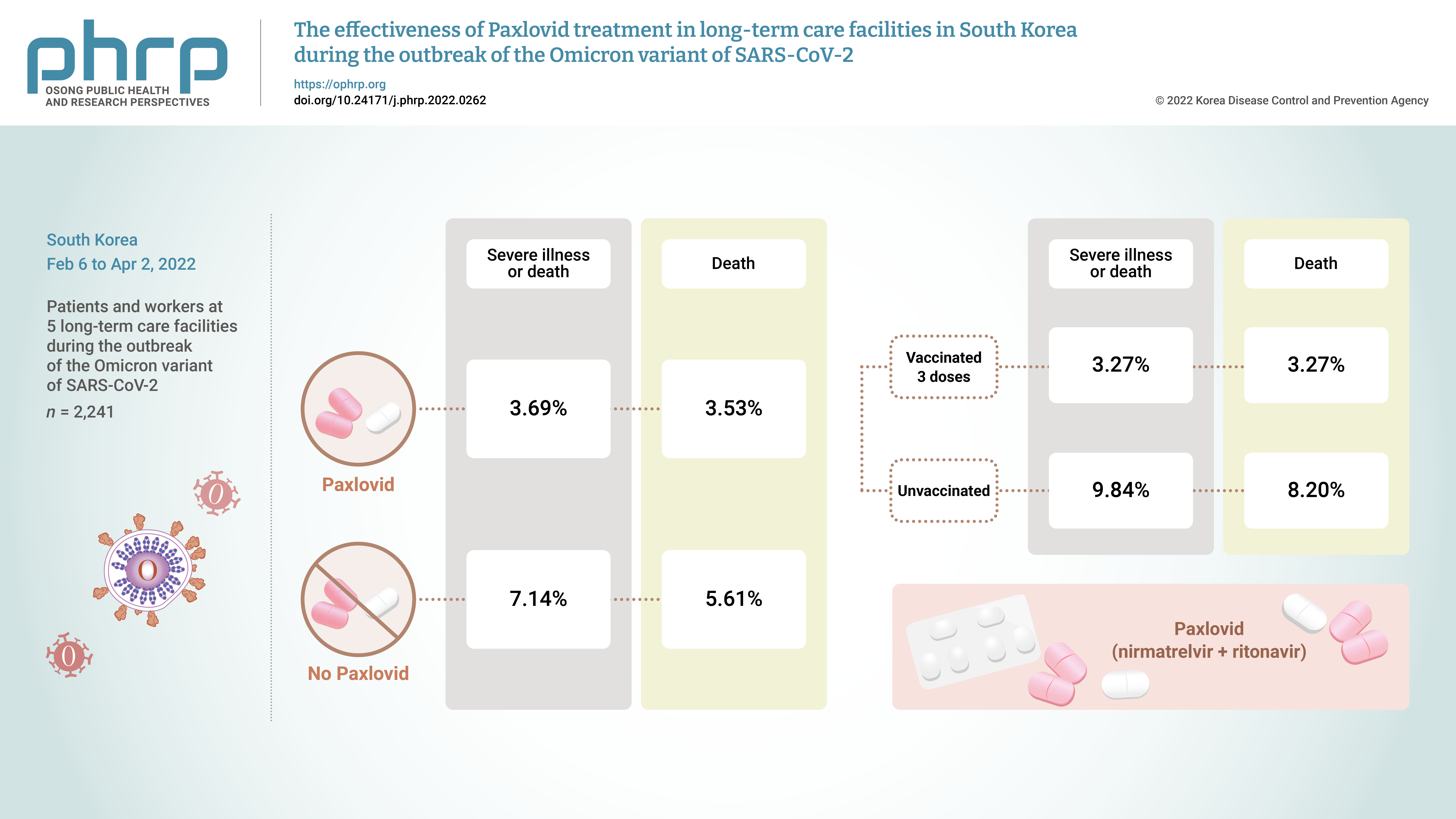
- From February 6, 2022 to April 2, 2022, the incidence of COVID-19 and the effects of treatment with Paxlovid were analyzed in 2,241 patients and workers at 5 long-term care facilities during the outbreak of the Omicron variant of severe acute respiratory syndrome coronavirus 2 in South Korea.
-
Results
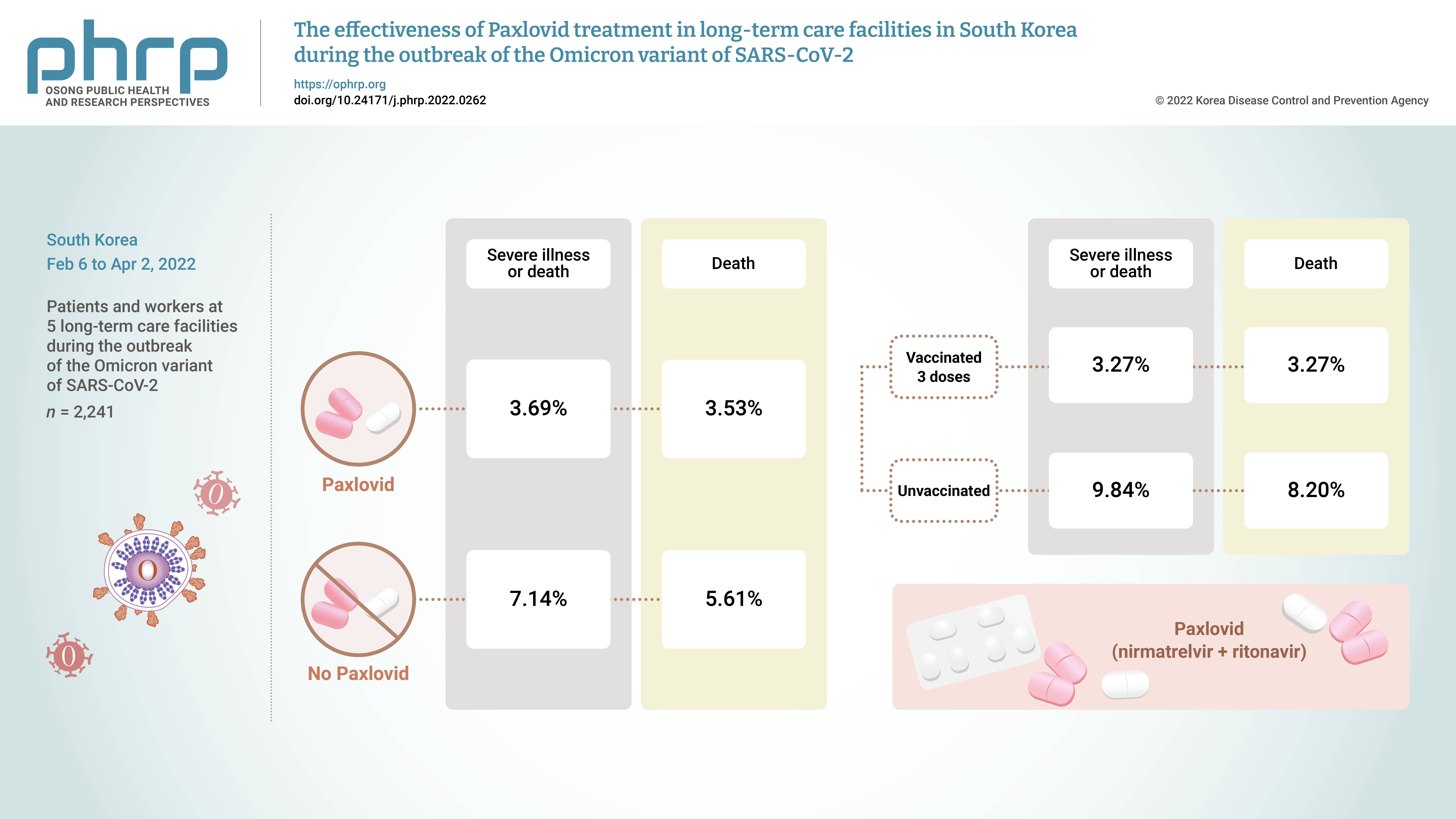
- The rate of severe illness or death in the group given Paxlovid was 51% lower than that of the non-Paxlovid group (adjusted risk ratio [aRR], 0.49; 95% confidence interval [CI], 0.24−0.98). Compared to unvaccinated patients, patients who had completed 3 doses of the vaccine had a 71% reduced rate of severe illness or death (aRR, 0.29; 95% CI, 0.13−0.64) and a 65% reduced death rate (aRR, 0.35; 95% CI, 0.15−0.79).
-
Conclusion
- Patients given Paxlovid showed a lower rate of severe illness or death and a lower fatality rate than those who did not receive Paxlovid. Patients who received 3 doses of the vaccine had a lower rate of severe illness or death and a lower fatality rate than the unvaccinated group.
-
Keywords: COVID-19; Effectiveness of vaccine; Omicron variant; Paxlovid
Graphical abstract
Introduction
- In November 2021, the World Health Organization reported the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which had the characteristics of immune evasion potential and fast transmission. Among other known variants of coronavirus disease 2019 (COVID-19), Omicron was classified as a major variant of concern [1,2]. Accordingly, to prepare for the spread of COVID-19 in Korea and other countries, epidemiological studies were actively conducted to identify the time required for diagnosis of the Omicron variant [3], explore the characteristics of its transmission in the community [4], and analyze the effects of the vaccine on the Omicron variant [5].
- By December 30, 2021, after the appearance of the Omicron variant in Korea, the vaccination rate increased along with the number of confirmed COVID-19 cases, as 82.7% of the general population had completed a second dose of the vaccine [6]. However, because the Omicron variant was easily transmitted, the cumulative number of confirmed cases in Korea as of April 25, 2022 reached 16,929,564, and the number of deaths per 100,000 population was 1.05 in the fourth week of February 2022. This rate increased to 1.74 in the first week of March 2022, to 2.61 in the second week of March 2022, and to 3.79 in the third week of March 2022 [7].
- Although the Omicron variant had a low severity rate in confirmed patients, those admitted to long-term care facilities (LTCFs) were more likely to have severe illness and death than those in the community, since most of them had underlying diseases. Therefore, treatment was necessary to prevent infections in this high-risk group. Early studies had recommended the development of a medication that could be administered orally and conveniently, along with the development of a vaccine to prevent COVID-19 [8].
- In November 2021, Pfizer Inc. announced Paxlovid (nirmatrelvir+ritonavir) as a medication that could reduce hospitalizations and deaths in patients with COVID-19. This medication was developed to inhibit the action of the SARS-CoV-2 proteolytic enzyme and was formulated as an oral treatment [9]. In addition, Pfizer reported that using Paxlovid could reduce the risk of hospitalization or death in confirmed patients by 89% [10].
- In Korea, the use of Paxlovid, was started on January 14, 2022 [11]. The target population for treatment included those over 60 years old, the immunocompromised, and those with underlying diseases in their 50s. Since the number of confirmed cases and deaths increased due to the prevalence of the Omicron variant, on February 21, 2022, the scope of treatment targets was expanded to include those with underlying diseases in their 40s [12]. As of March 3, 2022, the cumulative use of Paxlovid in Korea was 25,342 courses; specifically, 20,827 courses for those treated at home, 785 courses for people in community treatment centers, and 3,730 courses for people in hospitals specializing in infectious diseases [13]. Long-term care hospitals and facilities were at high risk for severe cases when confirmed cases of COVID-19 occurred among their patients.
- A systematic analysis to assess the effectiveness of major government measures, such as continuous monitoring of outbreaks and the use of vaccines and treatments, was needed. Therefore, this study investigated the incidence of COVID-19 in 5 LTCFs during the peak of the Omicron variant outbreak from February 6, 2022 to April 2, 2022. The preventive effect of the COVID-19 vaccine and the effect of treatment with Paxlovid on the development of severe illness were evaluated in the residents of these LTCFs.
Materials and Methods
- Participants
- The analysis targeted 2,241 residents and workers from 5 LTCFs in Korea where COVID-19 occurred from February 6, 2022 to April 2, 2022. The observation period was 52 days from the start of the Omicron outbreak to April 2, 2022, and the data for analysis were collected directly from each LTCF. The type of drug administered was determined by the medical staff, taking into consideration the patient's medical condition and current medications.
- Statistical Analysis
- This study used a retrospective cohort design, and the participants' general characteristics were presented as categorical variables using descriptive statistics. To compare severity and mortality according to the treatments and vaccines used, the relative risk was estimated and logistic regression analysis was applied. The term “severe cases” referred to patients with critical, life-threatening illness and those who died. The analysis model was adjusted for sex, age, vaccination history, and treatment history. All analyses were performed using R ver. 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria), and the results were presented with 95% confidence intervals (95% CIs).
- Ethics statement
- The study collected data in accordance with Article 76-2 of the Infectious Diseases Control and Prevention Act and was approved by the Institutional Review Board of the Korea Disease Control and Prevention Agency (IRB No: 2012-12-02-PE-A) of the Korea Disease Control and Prevention Agency.
Results
- The average incidence rate of COVID-19 in the LTCF groups was 71.9% (95% CI, 58.6%–86.2%). Among the confirmed cases, 44.7% (95% CI, 26.9%–63.0%) of residents and 0.2% (95% CI, 0%–1.80%) of workers received oral drug treatment, of which 86.8% (95% CI, 72.9%–100%) was Paxlovid, and 13.2% (95% CI, 12.4%–27.1%) was remdesivir (Veklury; Gilead Sciences Inc., Foster City, CA, USA) or Regkirona (regdanvimab; Celtrion Healthcare, Incheon, Korea). The number of severe cases ranged from 2 to 19, and the number of deaths from 0 to 18 in the 5 LTCFs. The detailed status of each LTCF is shown in Table S1–S4.
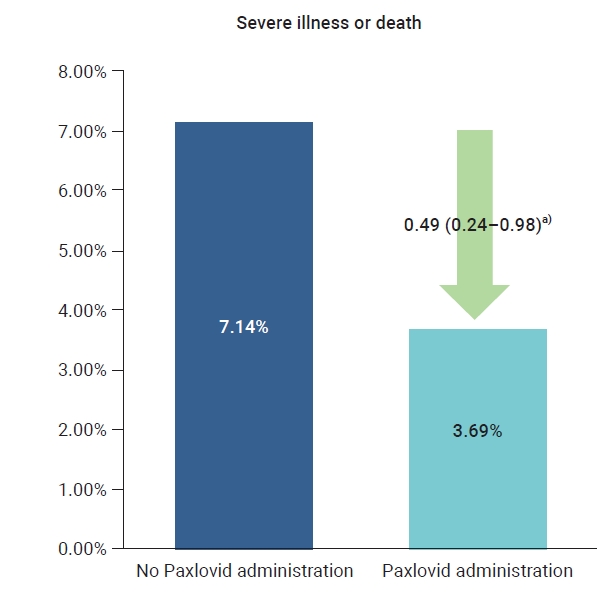
- The crude rate of severe cases was 3.7% for the residents who received Paxlovid and 7.1% for those who did not, and the crude mortality rate was 3.5% for those who received Paxlovid and 5.6% for those who did not. To compare the preventive effects of Paxlovid in residents who received the treatment compared to those who did not, the relative risk was estimated using logistic regression analysis and was adjusted for sex, age, and vaccination history. The adjusted rate of severe illness among residents who received Paxlovid was 51% lower than the rate among residents who did not receive Paxlovid (adjusted risk ratio [aRR], 0.49; 95% CI, 0.24−0.98) (Table 1; Figures 1, 2).
- Among the residents, the crude rate of severe disease was 9.84% for those who were not vaccinated and 3.27% for those who had completed 3 doses of the vaccine. Furthermore, the crude rate of mortality was 8.20% for the unvaccinated and 3.27% for those who had completed 3 doses of the vaccine. To compare the preventive effects of vaccination, the relative risks were estimated using logistic regression, adjusted for sex, age, and treatment history. For residents who received 3 doses of the vaccine, the adjusted rate of severe cases was 71% lower than in residents who were unvaccinated (aRR, 0.29; 95% CI, 0.13−0.64), and the rate of mortality was 65% lower than in the unvaccinated (aRR, 0.35; 95% CI, 0.15−0.79) (Figure S1, S2).
Discussion
- Our study analyzed the effect of treatments and vaccines on residents of LTCFs, where a cluster of Omicron variant outbreaks occurred from February 6 to April 2, 2022. Our results confirmed that patients treated with Paxlovid had a 51% lower rate of severe cases than those who did not receive Paxlovid treatment. In addition, residents who had received 3 doses of the vaccine had a 71% lower rate of severe cases and a 65% lower rate of mortality than those who were not vaccinated.
- Studies by Pfizer that targeted adults at risk of severe illness and death among non-hospitalized COVID-19 patients reported that the risk of hospitalization or death in these patients was reduced by 89% when they received Paxlovid within 3 to 5 days of symptom onset [9,10]. The monitoring period for hospitalization and mortality in the Pfizer study was 28 days. Furthermore, a study in Hong Kong reported that nirmatrelvir/ritonavir prevented hospitalization in 20% of confirmed COVID-19 patients [14]. Our study targeted patients hospitalized for confirmed COVID-19, and although there was a difference in that our dependent variables were critical disease and death, the effects of Paxlovid were confirmed.
- As of March 4, 2022, the quarantine authorities in Korea reported that the BA.2 variant, a subclassification of the Omicron variant, had become dominant [15]. The authorities promptly conducted risk assessments for the new variant as well as response effect analyses, with the intention of minimizing the negative impact of new variants on public health. They have periodically analyzed outbreaks, deaths, and vaccination effects in long-term care hospitals and facilities to identify the epidemiological characteristics and treatment effects in cluster outbreaks.
- Compared to the Delta variant, the Omicron variant had a lower fatality rate but a higher incidence rate [16], resulting in an increased number of severe cases and deaths along with an explosion of confirmed cases. Even though the effectiveness of the vaccine against the Omicron variant has been confirmed for Omicron mutations [5], to minimize severe illness and death, the intensive management and analysis of high-risk groups in vulnerable facilities should continue. In addition, the Central Quarantine Countermeasures Headquarters has distributed a guide for the correct use of COVID-19 treatments. Notably, medical staff administering Paxlovid must carefully consider underlying diseases and current medications when deciding whether to administer the drug [17].
- The main limitation of this study was that deaths due to other causes, which are characteristic of patients in LTCFs, could not be excluded. We did not adjust for the underlying diseases and conditions that could have affected the death rate among those being treated for COVID-19. However, we did consider that most of the subjects had comorbidities because they were patients in LTCFs. It is suggested that an extended sample of study subjects and a longer monitoring period be used in future studies to supplement the limitations of this study. This study is significant, however, because it is the first study on this topic to adjust for major factors related to death in residents of LTCFs in Korea, which have similar environments. Our findings confirm that the COVID-19 vaccine and Paxlovid effectively reduced the severity and fatality rates of the COVID-19 Omicron variant. In the future, it is expected that adverse reactions and treatment side effects will be investigated and that information will be used to help establish policies for COVID-19 treatment and prevention.
Supplementary Material
Table S1. Details of LTCF, from February 6, 2022 to April 2, 2022.; Table S2. General characteristic and incidence in subjects; Table S3. General characteristics of all patients at long-term care facilities according to therapeutic agent used; Table S4. Severity according to vaccination status among patients at five long-term care facilities; Figure S1. Comparison of severity according to vaccination history of patients of long-term care facilities with an outbreak of the Omicron variant, from February 6, 2022 to April 2 2022; Figure S2. Comparison of death according to vaccination history of patients of long-term care facilities with an outbreak of the Omicron variant, from February 6, 2022 to April 2, 2022. Supplementary data are available at https://doi.org/10.24171/j.phrp.2022.0262.
Table S3.
General characteristics of all patients at long-term care facilities according to therapeutic agent used
j-phrp-2022-0262-suppl3.pdf
Figure S1.
Comparison of severe illness or death according to vaccination history of patients of long-term care facilities with an outbreak of the Omicron variant, from February 6, 2022 to April 2, 2022. a)When non-treatment was set as reference, adjusted relative risk (95% confidence interval).
j-phrp-2022-0262-suppl5.pdf
Figure S2.
Comparison of death according to vaccination history of patients of long-term care facilities with an outbreak of the Omicron variant, from February 6, 2022 to April 2, 2022. a)When non-treatment was set as reference, adjusted relative risk (95% confidence interval).
j-phrp-2022-0262-suppl6.pdf
Article information
-
Ethics Approval
This study was approved by the Institutional Review Board of the Korea Disease Control and Prevention Agency (IRB No: 2012-12-02-PE-A) and conducted in accordance with the principles of the Declaration of Helsinki.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available. If you have any question about this study, contact the corresponding author (pahmun@korea.kr)
-
Authors’ Contributions
Conceptualization: YJP; Data curation: HP, HYL, MY, JJL, ESL, YK; Formal analysis: HP, HYL, MY; Methodology: YJP, HP, SEL, HYL, MY, YJS; Project administration: HP; Visualization: YJP, HP; Writing-original draft: HP; Writing-review & editing: all authors.
Acknowledgements- The authors appreciate the long-term care facilities for making this study possible.
Figure 1.Comparison of severe illness or death according to the Paxlovid administration history in patients at 5 Korean long-term care facilities during the Omicron variant outbreak, from February 6, 2022 to April 2, 2022. a)When non-treatment was set as reference, adjusted relative risk (95% confidence interval).
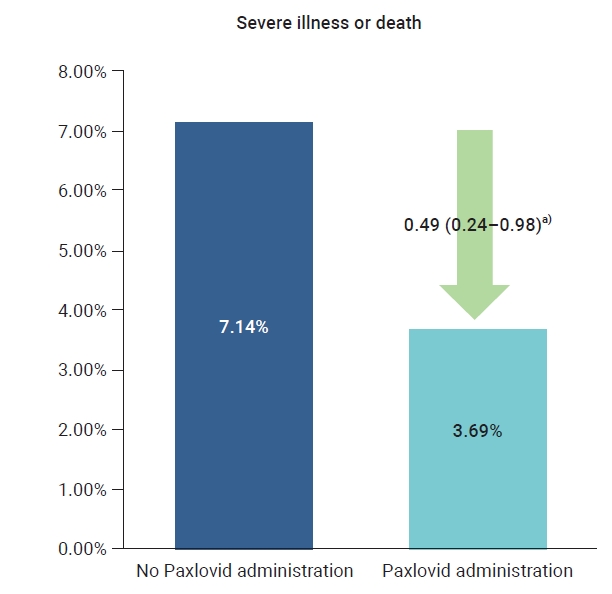
Figure 2.Comparison of death according to the Paxlovid administration history in patients at 5 Korean long-term care facilities during the Omicron variant outbreak, from February 6, 2022 to April 2, 2022. a)When non-treatment was set as reference, adjusted relative risk (95% confidence interval).
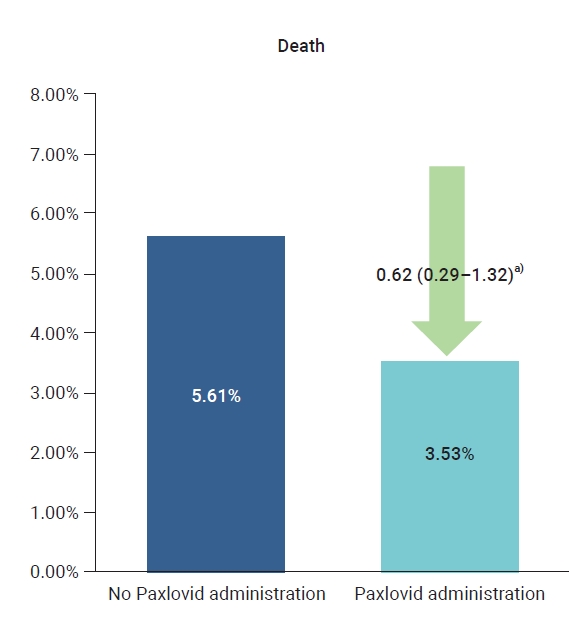
Table 1.Severity of COVID-19 (Omicron variant) according to Paxlovid use among all patients at 5 Korean long-term care facilities
|
Variable |
Total |
Severe illness or death
|
Death |
|
Total (%) |
cRR (95% CI) |
aRR (95% CI) |
Total (%) |
cRR (95% CI) |
aRR (95% CI) |
|
Total |
819 |
37 (4.5) |
|
|
33 (4.0) |
|
|
|
No treatment |
196 |
14 (7.1) |
1.00 (ref.) |
1.00 (ref.) |
11 (5.6) |
1.00 (ref.) |
1.00 (ref.) |
|
Treated with Paxlovid |
623 |
23 (3.7) |
0.50 (0.25−0.99) |
0.49 (0.24−0.98) |
22 (3.5) |
0.62 (0.29−1.29) |
0.62 (0.29−1.32) |
References
- 1. World Health Organization (WHO). Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern [Internet]. Geneva: WHO; 2021 [cited 2022 Sep 29]. Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- 2. World Health Organization (WHO). Tracking SARS-CoV-2 variants [Internet]. Geneva: WHO; 2021 [cited 2021 Nov 26]. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
- 3. Lee HR, Choe YJ, Jang EJ, et al. Time from exposure to diagnosis among quarantined close contacts of SARS-CoV-2 Omicron variant index case-patients, South Korea. Emerg Infect Dis 2022;28:901−3.ArticlePubMedPMC
- 4. Kim EY, Choe YJ, Park H, et al. Community Transmission of SARS-CoV-2 Omicron Variant, South Korea, 2021. Emerg Infect Dis 2022;28:898−900.ArticlePubMedPMC
- 5. Kim J, Choe YJ, Jang EJ, et al. Effectiveness of booster mRNA vaccines against SARS-CoV-2 infection in an elderly population, South Korea, October 2021-January 2022. Clin Infect Dis 2022;75:920−1.ArticlePubMedPDF
- 6. Korea Disease Control and Prevention Agency (KDCA). Press release. The third doses of vaccinations is 74.7% of those aged 60 or older in December [Internet]. Cheongju: KDCA; 2021 [cited 2022 Sep 29]. Available from: http://ncov.mohw.go.kr/upload/viewer/skin/doc.html?fn=1640309879513_20211224103759.pdf&rs=/upload/viewer/result/202206/. Korean.
- 7. Korea Disease Control and Prevention Agency (KDCA). Press release. COVID-19 [Internet]. Cheongju: KDCA; 2022 [cited 2022 Sep 29]. Available from: http://ncov.mohw.go.kr/upload/viewer/skin/doc.html?fn=1650846858732_20220425093419.pdf&rs=/upload/viewer/result/202206/. Korean.
- 8. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann Med 2022;54:516−23.ArticlePubMedPMC
- 9. Pfizer Inc. Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study [Internet]. Pfizer Inc.; 2021 [cited 2022 Sep 29]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate.
- 10. Mahase E. Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 2021;375:n2713. ArticlePubMed
- 11. Korea Disease Control and Prevention Agency (KDCA). Press release. The paxlovid for home care cases administration begins [Internet]. Cheongju: KDCA; 2022 [cited 2022 Jun 2]. Available from: https://ncov.kdca.go.kr/SynapDocViewServer/viewer/doc.html?key=5c524b8bbe6b4211be79500a26c74c7e&convType=img&convLocale=en_US&contextPath=/SynapDocViewServer. Korean.
- 12. Korea Disease Control and Prevention Agency (KDCA). Press release. Weekly risk assessment is high across the country, the metropolitan area, and the non-metropolitan area [Internet]. Cheongju: KDCA; 2022 [cited 2022 Jun 2]. Available from: https://ncov.kdca.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6409&contSeq=6409&board_id=312&gubun=ALL#. Korean.
- 13. Korea Disease Control and Prevention Agency (KDCA). Press release. COVID-19 outbreak and vaccination status in Korea [Internet]. Cheongju: KDCA; 2022 [cited 2022 Jun 2]. Available from: https://www.kdca.go.kr/filepath/boardDownload.es?bid=0015&list_no=718890&seq=2. Korean.
- 14. Yip TC, Lui GC, Lai MS, et al. Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients. Clin Infect Dis 2022;ciac687.
- 15. Korea Disease Control and Prevention Agency (KDCA). Press release. The number of new occurrence in COVID-19 week [Internet]. Cheongju: KDCA; 2022 [cited 2022 Jun 2]. Available from: https://www.korea.kr/common/download.do?fileId=196753782&tblKey=GMN. Korean.
- 16. Park H, Lee JJ, Choi JH, et al. Incidence and fatality rates of SARS-CoV-2 Omicron variant compared with Delta variant in long term care facilities. Public Health Wkly Rep 2022;15:1426−34.
- 17. Korea Disease Control and Prevention Agency (KDCA). Guide to use of coronavirus infectious diseases-19 treatment [Internet]. Cheongju: KDCA; 2022 [cited 2022 Sep 29]. Available from: https://www.kdca.go.kr/upload_comm/syview/doc.html?fn=164940956584400.pdf&rs=/upload_comm/docu/0019/. Korean.
Citations
Citations to this article as recorded by

- Efficacy and safety of antiviral treatments for symptomatic COVID-19 outpatients: Systematic review and network meta-analysis
Meital Zur, Thalia Peselev, Stav Yanko, Victoria Rotshild, Ilan Matok
Antiviral Research.2024; 221: 105768. CrossRef - Clinical Effectiveness of Ritonavir-Boosted Nirmatrelvir—A Literature Review
Sydney Paltra, Tim O. F. Conrad
Advances in Respiratory Medicine.2024; 92(1): 66. CrossRef - Effectiveness of nirmatrelvir‐ritonavir on severe outcomes of COVID‐19 in the era of vaccination and Omicron: An updated meta‐analysis
Sien Ombelet, Diego Castanares‐Zapatero, Fabian Desimpel, Frank Hulstaert, Sabine Stordeur, Dominique Roberfroid
Journal of Medical Virology.2024;[Epub] CrossRef - COVID‐19 infection in patients with haematological malignancies: A single‐centre survey in the latest Omicron wave in China
Xiaolu Zhu, Qian Jiang, Jin Lu, Yuqian Sun, Xiaosu Zhao, Shenmiao Yang, Feifei Tang, Wenjing Yu, Ting Zhao, Xiaohong Liu, Jinsong Jia, Wenbing Duan, Lijuan Hu, Jing Wang, Yang Liu, Nan Peng, Xuelin Dou, Rui Ma, Qiang Fu, Huifang Wang, Kaiyan Liu, Xiaojun
British Journal of Haematology.2023; 202(1): 31. CrossRef - The association mental health of adolescents with economic impact during the COVID-19 pandemic: a 2020 Korean nationally representative survey
Hanul Park, Kang-Sook Lee
BMC Public Health.2023;[Epub] CrossRef - Efficacy and safety of paxlovid (nirmatrelvir/ritonavir) in the treatment of COVID‐19: An updated meta‐analysis and trial sequential analysis
Haokun Tian, Changsen Yang, Tiangang Song, Kechen Zhou, Lequan Wen, Ye Tian, Lirui Tang, Weikai Xu, Xinyuan Zhang
Reviews in Medical Virology.2023;[Epub] CrossRef - Real-World Effectiveness of Nirmatrelvir-Ritonavir and Its Acceptability in High-Risk COVID-19 Patients
Min-Kyung Kim, Kyung-Shin Lee, Sin Young Ham, Youn Young Choi, Eunyoung Lee, Seungjae Lee, Bora Lee, Jaehyun Jeon, BumSik Chin, Yeonjae Kim, Gayeon Kim, Hee-Chang Jang, Jae-Phil Choi, Sang-Won Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score Matched Study
Hye Rim Park, Min-Gyu Yoo, Jong Mu Kim, Soon Jong Bae, Hyungmin Lee, Jungyeon Kim
Infection & Chemotherapy.2023; 55(4): 490. CrossRef - Nirmatrelvir combined with ritonavir for preventing and treating COVID-19
Stefanie Reis, Maria-Inti Metzendorf, Rebecca Kuehn, Maria Popp, Ildiko Gagyor, Peter Kranke, Patrick Meybohm, Nicole Skoetz, Stephanie Weibel
Cochrane Database of Systematic Reviews.2023;[Epub] CrossRef
 , Young Joon Park1
, Young Joon Park1 , Hye Young Lee1
, Hye Young Lee1 , Mi Yu1
, Mi Yu1 , Yeong-Jun Song1
, Yeong-Jun Song1 , Sang Eun Lee1
, Sang Eun Lee1 , Ji-Joo Lee2
, Ji-Joo Lee2 , Eun-Sol Lee2
, Eun-Sol Lee2 , Yeonjung Kim2
, Yeonjung Kim2






 Cite
Cite