Impact of fatigue on quality of life among breast cancer patients receiving chemotherapy
Article information
Abstract
Objectives
Fatigue is the most frequently reported symptom experienced by cancer patients and has a profound effect on their quality of life (QOL). The study aimed to determine the impact of fatigue on QOL among breast cancer patients receiving chemotherapy and to identify the risk factors associated with severe fatigue incidence.
Methods
This was an observational prospective study carried out at multiple centers. In total, 172 breast cancer patients were included. The Functional Assessment of Chronic Illness Therapy-Fatigue Questionnaire was used to measure QOL, while the Brief Fatigue Inventory (BFI) was used to assess the severity of fatigue.
Results
The total average mean and standard deviation of QOL were 84.58±18.07 and 4.65±1.14 for BFI scores, respectively. A significant association between fatigue and QOL was found in linear and multiple regression analyses. The relationships between fatigue severity and cancer stage, chemotherapy dose delay, dose reduction, chemotherapy regimen, and ethnicity were determined using binary logistic regression analysis.
Conclusion
The findings of this study are believed to be useful for helping oncologists effectively evaluate, monitor, and treat fatigue related to QOL changes.
Introduction
Breast cancer is the most common malignancy among women globally, particularly among women over the age of 40 [1]. In Malaysia, breast cancer is the most commonly diagnosed cancer in women, followed by colorectal cancer, and it is the second leading cause of cancer death among Malaysians after colorectal cancer [2]. Cancer-related fatigue (CRF) is a multidimensional concept reported to be the most common side effect experienced by cancer patients [3]; it has a profound negative effect on patients' quality of life (QOL) [4]. The fatigue reported by cancer patients is usually described as an unusual, excessive, whole-body experience that is unrelated to activity or exertion and is not relieved by rest or sleep [5,6]. The prevalence of fatigue among cancer patients has been estimated to be between 10% to 99% [7] and reaches as high as 90.3% among patients receiving chemotherapy [8], with a marked effect on patients’ QOL [4,9]. Most studies have reported relatively high rates of moderate to severe fatigue (30%−60%), which may lead to treatment discontinuation [10]. It has been found that the QOL among cancer patients is significantly and negatively affected from the moment of diagnosis with cancer or once they hear the word “cancer” [11]. A study of the scientific literature demonstrated a strong relationship between fatigue and poor QOL in cancer patients [12]. There is a growing interest in using QOL data in clinical studies to help clinicians improve health services among cancer survivors [13]. Recently, QOL has been introduced as a point of comparison between treatments for many types of cancer, particularly in advanced stages [14]. QOL has also been used as an early indicator of disease progression that could help oncologists and healthcare providers more closely monitor patients in their daily routines [15].
Several factors play a significant role in the incidence of severe fatigue in cancer patients. The predominant factors are demographic characteristics such as obesity [16−18], pre-menopausal status [18], age [17,19,20], and level of education [17,20]. In addition, the development of severe fatigue is associated with pathological factors such as the presence of interleukin (IL)-6, tumor necrosis factor, IL-1 receptor antagonist (IL-1RA), and especially IL-8, which is a significant factor related to pain and fatigue in cancer patients [21], the types of cancer [19], and anticancer treatment schedules [22−24]. Still other factors include illness-related characteristics (pain, inflammation, and joint damage), physical function (sleep disturbance and disability), emotional impairment (depression and anxiety), and personal conditions (gender, work, social relationships, education, and whether the patient has a partner) [25]. In addition, patient-related factors, treatment-related factors, inflammatory cytokines, and metabolic and/or endocrine dysregulation play significant roles in the incidence and/or severity of fatigue among breast cancer patients [26,27].
Improving the QOL of cancer patients is expected to have significant benefits for their adaptation and motivation to continue receiving and completing chemotherapy with fewer side effects and to increase the chances of cancer patients being cured and surviving [28]. Hence, this study aimed to determine the effect of CRF on the QOL of Malaysian breast cancer patients and to detect the risk factors associated with fatigue severity. The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale was used to assess the impact of fatigue on QOL, and the Brief Fatigue Inventory (BFI) scale was used to measure fatigue severity among breast cancer patients. The outcome of this study will significantly help improve the techniques used by healthcare providers and medical staff to care for cancer patients. Future studies that focus on improving the QOL of cancer patients are highly recommended because such research will help cancer patients to overcome the disease itself [29−31].
Materials and Methods
This prospective observational multi-center study was carried out at the oncology department and daycare clinics of Institute Kanser Negara, Putrajaya, Hospital Kuala Lumpur, and University of Malaya Medical Center, Malaysia, from July 2019 to April 2020. Patients were recruited based on the following criteria: (1) being diagnosed with breast cancer at any stage and receiving chemotherapy; (2) not having received radiation treatment and hormonal therapy; (3) having a fatigue score of 2 or above after cancer diagnosis based on the Eastern Cooperative Oncology Group Performance Status (ECOG PS) and BFI; (4) not having chronic diseases, such as hypertension, renal disease, cardiac disease, and diabetes mellitus, or being in a poor psychological state; (5) being able to complete the questionnaire unaided; and (6) being aged above 18 years old. Patients having any other malignancy and patients with an ECOG PS of 0−1 were excluded from the study.
Patients who experienced fatigue were identified based on the ECOG PS and BFI scores, which is used to determine the prognostic status of fatigue, as recorded in their medical files. The severity of fatigue was assessed by the BFI scale: 0−3 (mild), 4−7 (moderate), and 7−10 (severe) [32]. The patients were then briefed on the background of the study and given a consent form to sign once they agreed to participate. Prior permission was granted by the owners of the FACIT system to use their tool in this study. The test was conducted and scored using the instructions from version 4 of the FACIT Measurement System (www.facit.org). Patients were followed up on the same day after receiving chemotherapy to complete the FACIT-F (version 4) questionnaire in English and/or Malay language format (whichever was needed) and the BFI scale. The respondents were required to circle or mark 1 number per line to indicate their responses as they applied to the past 7 days. The response scale ranged from 0 (not at all) to 4 (very much). In addition, patients were instructed to answer the first 3 questions from the BFI scale to indicate the severity of fatigue during the previous 24 hours. These patients were followed up with for another cycle of chemotherapy (totaling 2 cycles). With the aid of the medical staff, the BFI and FACIT-F QOL assessment were completed during each follow-up. It took about 10 to 20 minutes to complete the questionnaires. The 2 follow-up visits for fatigue patients were performed within 2 to 4 months.
Tools for Measurements and Procedure
Brief Fatigue Inventory
The BFI is a tool defined to assess the severity of fatigue in cancer patients over the previous 24 hours (Cronbach’s alpha, 0.82-0.97) [33]. The BFI is also used to screen, detect, and measure the severity of daily fatigue. It has many advantages such as easily understandable language, simplicity, and a short completion time (up to 10 minutes), which makes it one of the best tools for measuring fatigue among cancer patients. Originally the BFI was used for patients who speak English, but it is now available and used in many other languages [34,35]. The BFI consists of 9 questions answered using an 11-point rating scale. The severity of fatigue is rated according to the first 3 questions (1. Rate your level of fatigue now?, 2. Rate your USUAL level of fatigue during the past 24 hours?, 3. Rate your WORST level of fatigue during the past 24 hours?) on a scale of 0 to 10, with 0 indicating no fatigue and 10 indicating the most severe level of fatigue. The next 6 questions are used to assess and detect the interference of fatigue with aspects of a patient’s daily life such as the ability to walk, their mood, ability to work, their enjoyment of life, and connections with other people. In this study, only the first 3 questions were used to assess the severity of fatigue among breast cancer patients.
The Functional Assessment of Chronic Illness Therapy-Fatigue scale
The FACIT-F scale is a tool designed specifically to assess the impact of fatigue on QOL. The FACIT-F questionnaire consists of 40 items. Twenty-seven items are derived from version 4 of the Functional Assessment of Cancer Therapy-General (FACT-G), which contains 4 QOL domains: physical well-being (PWB), social well-being (SWB), emotional well-being (EWB), and functional well-being (FWB). The other 13 items are from the fatigue subscale (FS) [36]. Each question had an equal value and the QOL was quantified as the sum of the scores for all domains. The QOL domains scores were classified into 5 categories, which were ‘not at all’ (0), ‘a little bit’ (1), ‘somewhat’ (2), ‘quite a bit’ (3), and ‘very much’ (4). Patients were followed up and assessed during each of their chemotherapy cycles, starting from cycle 2, which varied from 3 to 4 weeks depending on the type of chemotherapy treatment that they received.
FACIT-F scoring
The FACIT-F (version 4) contains 40 self-reported items, including 27 items from the core FACT-G scale and 13 items from the additional FS.
FACT-G 27 items = PWB 7 items + SWB 7 items + EWB 6 items + FWB 7 items
FS 13 items = Fatigue 13 items
FACIT-F 40 items = FACT-G 27 items+ FS 13 items
The final score is obtained by adding the scores from the 4 FACT-G domains (PWB, SWB, EWB, and FWB) to the score from the additional FS (13 items). The final score for fatigue patients (FACIT-F) ranges from 0 to 160 (Table 1). Negatively stated items are reversed by subtracting the response from 4. After reversing the necessary items, all subscale items are tabulated, the result of which is the subscale score.
Subscale scores are prorated if more than 50% of the items are answered (e.g., a minimum of 4 of 6 items or 4 of 7 items) and any number of unanswered or missing questions remained. This is done using the following formula:
prorated subscale score = (sum of item scores) × (no. of items in subscale) / (no. of items answered)
The total score is then calculated as the sum of the subscale scores. The FACIT-F scale is considered to be an acceptable indicator of the QOL among cancer patients as long as the overall item response rate was greater than 80%. To achieve this result, at least 22 of 27 FACT-G items must be completed [36]. High scores for all FACIT scales and symptom indices have been associated with higher health-related QOL [37]. Higher scores on the FACIT-F have been shown to correlate with lower BFI scores and better QOL among cancer patients [38].
Statistical Analysis
The mean and standard deviation (SD) were used as descriptive statistics to present the demographic and clinical data in this study. Regression analysis was performed. Both simple and multiple regression analyses with Pearson correlation coefficients were conducted to determine the effect and association between the total average of BFI scores and total average QOL (FACIT-F) scores, as well as between the total average BFI scores and the corresponding total average QOL domain subgroup. Logistic regression analysis was used to determine the relationship between fatigue severity and its associated factors such as cancer stage, chemotherapy data, and demographic characteristics. The dependent variables in all models were the QOL and BFI scores, and the independent variables were CRF, as well as other factors such as age, cancer stage, marital status, body mass index, chemotherapy types, dose delay, and the number of chemotherapy regimens. A relationship was considered statistically significant if the p was <0.05. Data were analyzed using IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). Computation of the results was done according to the guidelines of the FACT-G group. In all analyses, the first measurement was taken as a reference baseline level and changes relative to the baseline measurement (in follow-up visits after cycle 1) were analyzed. The effect of fatigue on QOL among breast cancer patients receiving chemotherapy was represented by interaction terms between the variables of fatigue (BFI severity) and QOL at the second follow-up. The association between fatigue severity (BFI scores) from the first follow-up (the first reading taken on the same day of receiving chemotherapy) and upcoming assessments (the second reading) were calculated separately for these scales. In addition, changes in the mean value between both readings were also calculated. Multiple logistic regression analysis was conducted to determine the relationships between cofactors and the fatigue score.
Results
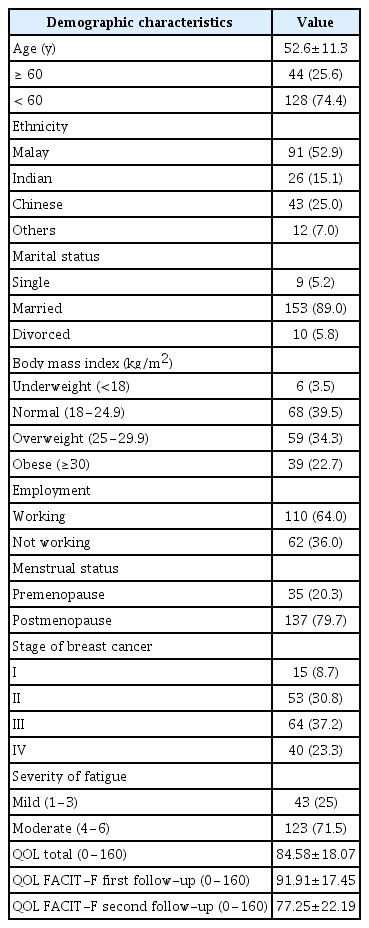
Out of 172 respondents, 91 of them (52.9%) were Malay. The majority of respondents (n=128; 74.4%) were <60 years old with a mean age of 52.63 years (SD, 11.27 years). The majority of the respondents were married (n=153, 89%) and more than half of the respondents were working (n=110, 64%). Sixty-four respondents (37.2%) had stage III breast cancer, and 53 respondents (30.8%) had stage II cancer. The distribution of demographic characteristics such as ethnicity, age, marital status, menstrual status, employment status, smoking status, and alcohol consumption is reported in Table 2.
Fatigue Severity
Based on the BFI, fatigue severity was classified into 3 main categories: mild (1−3), moderate (4−6), and severe (7−10) [32]. Table 2 shows that most of the patients suffered from moderate fatigue (n=123, 71.5%) followed by mild fatigue (n=43, 25%).
QOL Data
The average mean score and SD for total QOL was 84.58±18.07. The mean QOL score at the first follow-up (91.91±17.45) was higher than the mean QOL score at the second follow-up (77.25±22.19). The scores for total QOL clearly declined across the two follow-up visits, as shown in Table 2.
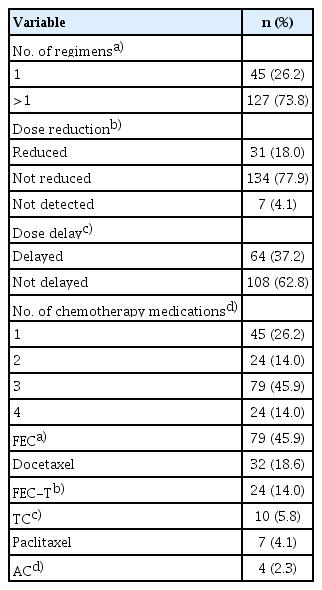
Chemotherapy Data
The majority of the patients (n=127, 73.8%) received a combination of chemotherapy regimens, and the rest (n=45, 26.2%) received only 1 regimen. In addition, 79 patients (45.9%) were prescribed fluorouracil, epirubicin, cyclophosphamide (FEC); 32 patients (18.6%) were prescribed with docetaxel; and 24 patients (14%) were prescribed with a combination regimen of FEC and docetaxel (FEC-T), as shown in Table 3.
The Severity of Fatigue according to BFI Score across the 2 Follow-up Visits
The average mean of the total BFI was 4.65±1.14. Most respondents reported moderate fatigue at the 2 follow-up visits (n=121, 70.3%; and n=117, 68%), followed by mild fatigue and severe fatigue.
Association between Total QOL and BFI Scores
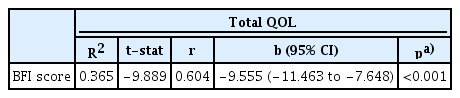
Linear regression analysis showed a significant (p<0.005) association at all follow-up visits between QOL and fatigue severity. Pearson correlation analysis revealed a moderate positive relationship between QOL and BFI scores regarding fatigue severity (r=0.604). As the severity of fatigue increased, the QOL score dropped to a greater extent and vice versa. Linear regression was calculated to predict the total QOL FACIT-F score based on fatigue severity. A significant regression equation was found (F (1,170)=97.789, p<0.001, R2=0.365). The equation for predicting QOL followed the format y=a+bx [39], where y=QOL (dependent variable), a=constant, b=BFI constant, x=BFI score (independent variable), the participants’ predicted QOL was equal to 128.993−9.555 (BFI score). In other words, the QOL score decreased by 9.555 units for each 1-unit increase in fatigue severity, as shown in Table 4. Additionally, as QOL decreased, fatigue severity (BFI score) increased, confirming an inverse relationship between both variables. The scatter plot also showed that QOL declined from the first follow-up to second follow-up, whereas BFI scores increased (Figure 1).
Association between QOL and BFI Scores in Both Follow-up Visits
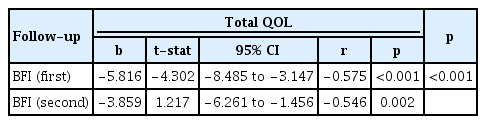
Multiple regression was carried out to investigate the relationship between the total QOL change and BFI scores at both follow-up visits and to predict the total QOL based on the BFI scores. The scatter plot showed a moderate negative linear relationship between the 2 variables, as confirmed by the Pearson correlation coefficient (r=0.575 at first follow-up and r=0.546 at second follow-up). Multiple regression showed a significant relationship between QOL across different domains and the BFI score (p<0.005). According to a predictive equation with the format y=a+bx1+bx2, where y=QOL (dependent variable), a=constant, b=BFI constant, x=BFI score (independent variable), the participants’ predicted QOL was equal to 128.730−5.816 (BFI score) for the first follow-up and 128.730−3.859 (BFI score) for the second follow-up. The QOL scores decreased by 5.816 units for each 1-unit increase in the BFI score at the first follow-up and declined by 3.859 units for each 1-unit increase in BFI score at the second follow-up (Table 5).
Association between Fatigue Severity and Its Associated Risk Factors
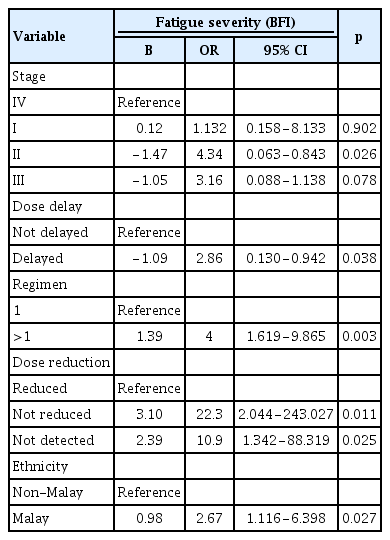
A logistic binary regression test was used to determine the strength of the association between fatigue severity and risk factors, incorporating all variables with significance according to the chi-square test and performing logistic binary regression once for all factors. As shown in Table 6, cancer stage (p=0.026), chemotherapy dose delay (p=0.038), chemotherapy regimen (p=0.003), chemotherapy dose reduction (p=0.011), and ethnicity (p=0.027) significantly predicted the magnitude of fatigue severity among breast cancer patients. There was a significant (p<0.005) negative relationship between cancer stages (specifically stage II) with fatigue severity. Hence, as the cancer stage increased, the severity of fatigue also increased. Participants who experienced dose delay were 2.86 times less likely to experience severe fatigue compared to those with no chemotherapy dose delay. In addition, breast cancer patients who received a combination of chemotherapy regimens were 4 times more likely to develop a higher level of fatigue than those who took only a single chemotherapy regimen. Moreover, breast cancer patients with no chemotherapy dose reduction were 22.3 times more likely to experience severe fatigue compared to those with a dose reduction. Finally, Malay patients were 2.6 times more likely to develop severe fatigue than non-Malay patients (Table 6).
Discussion
CRF is a highly prevalent phenomenon in individuals with cancer who receive anti-neoplastic chemotherapy, radiation therapy, or biological response modifiers [40]. Almost every patient suffers from fatigue during cancer treatment, with a prevalence rate of up to 99% [41]. Despite growing evidence regarding fatigue occurring due to various anticancer treatments and how CRF affects patients’ QOL, the severity of CRF is still not widely evaluated for cancer patients who are suffering from this distressing symptom. This study aimed to detect the factors associated with the severity of fatigue and how they affected QOL among cancer patients. To the best of the authors’ knowledge, this is the first prospective longitudinal study conducted at multiple centers for cancer treatment in Malaysia with these objectives. The results showed that fatigue showed a moderate correlation with reduced overall QOL. This finding is similar to that of a recent study done in Malaysia in 2018 [30] and several studies conducted globally, which reported significant negative relationships between QOL and fatigue [38,42−50]. The majority of cancer patients reported that fatigue prevented them from engaging in their normal daily routine and partaking in social activities such as spending time with friends or going to a restaurant [51]. Fatigue was also associated with a wide range of symptoms consistent with psychological impairment, including lack of motivation, depression, cognition, and mood disturbance [52,53].
The results also revealed a moderate negative relationship between QOL and BFI scores. This finding is consistent with the findings of Butt et al. [54], who reported a strong significant correlation between fatigue and QOL. Despite the well-known negative effect of fatigue on the QOL of cancer patients, it is distressing to realize from this study that it remains a persistent problem that worsens patients’ condition, significantly impacting their overall health status and the degree to which they experience physical symptoms. Cancer patients often describe their fatigue as a lack of energy, which could indicate that fatigue is caused by changes in metabolism and energy generation, particularly in skeletal muscles. In addition, fatigue might occur due to chemotherapy, which also exacerbates and induces various side effects that in turn negatively influence a patient’s QOL. Furthermore, fear of metastasis and disappointment from treatment may result in a diminished QOL [55].
Our data also indicated a significant association between fatigue severity and QOL domains at both follow-up visits. The QOL scores decreased by 5.816 units for each 1-unit increase in the BFI score for the first follow-up and declined by 3.859 units for each 1-unit increase in BFI score at the second follow-up. In other words, about 36.8% of QOL was explained by fatigue BFI scores at both follow-up visits. A significant association was found between BFI scores and all QOL FACIT-F scales, indicating that higher BFI scores were associated with lower QOL. This finding was supported by many studies reporting that patients who demonstrated greater fatigue intensity showed lower QOL [38,56,57]. A similar conclusion was drawn by 2 large survey-based studies, each involving more than 1,900 breast cancer patients, which reported an inverse relationship between higher CRF and lower physical and social functioning with regard to QOL [42,58,59]. Understanding why CRF adversely impacts QOL could be achieved through a deeper understanding of the experiences of cancer survivors. The negative impact of fatigue on QOL did not only reduce patients’ physical activity, but also affected them emotionally and functionally. Pappalardo and Reggio [60] found that fatigue diminished quality of life by interfering with the daily activities of patients. The study mentioned that 80% of fatigue patients lost their jobs (QOL, SWB) and reported a lack of ability to perform social tasks, work, and activities and to maintain a normal life [60]. Cancer patients often described their fatigue as a lack of energy, which suggested that fatigue resulted from changes in metabolism and energy generation, particularly in skeletal muscles [61]. In addition, patients undergoing chemotherapy often develop anorexia and reduce food intake, which could reduce their energy availability and contribute to fatigue [62].
There are several factors associated with the incidence of severe fatigue among cancer patients. In this study, the results showed a significant association between ethnicity (Malay) and CRF severity. This finding aligns with a previous study that revealed a significant relationship between the severity of fatigue and ethnicity among breast cancer survivors [17]. Hoh et al. [63] showed in their trial that the high association between the ethnicity of patients, particularly Malay patients, and fatigue severity might be due to the fact that Malays constitute the predominant racial group in Malaysia. The data also demonstrated a statistically significant (p<0.005) association between fatigue severity and breast cancer stage. The intensity of CRF increased at higher breast cancer stages. According to Savina and Zaydiner [4], the 2 main factors that significantly affected the incidence and intensity of CRF among cancer patients were cancer stage and cancer status. The intensity of fatigue was hypothesized to be related to the level of disease burden rather than different fatigue profiles, such as the relationship between physical and mental health. The proposed explanation for this finding was that, due to a higher concentration of cancer cells in the body of patients with advanced-stage cancer, the condition of cancer itself causes patients to feel tired. Patients with advanced-stage cancer also eat less, receive more intense doses of chemotherapy, and are less active [64].
In addition, the data in this study showed that most cancer patients who received combination chemotherapy regimens developed moderate to severe fatigue. The logistic regression analysis demonstrated that breast cancer patients who received combination regimens were 4 times more likely to develop a high level of fatigue than those who received a single regimen. This finding is in agreement with a previous trial, which reported that breast cancer patients who received combinations of chemotherapy medications experienced more severe fatigue than those who received only 1 chemotherapy medication (paclitaxel) [65]. A similar finding was reported by Abrahams et al. [66], according to whom the prevalence of severe fatigue increased from 7% to 52% after patients received a combination of chemotherapy regimens. Another trial reported that patients who received a combination chemotherapy regimen (cyclophosphamide, fluorouracil, adriamycin [doxorubicin], and/or docetaxel) experienced more severe fatigue than those who received only Taxol (paclitaxel) [65]. Combined therapy regimens with 2 or more chemotherapy medications exacerbate fatigue more than any medication taken on its own. Fatigue tends to worsen with subsequent cycles of chemotherapy, which suggests a cumulative dose-related toxic effect that affects QOL.
A significant association was also found between dose reduction and the severity of fatigue. This finding is supported by Wang et al. [67], who pointed out that hematological, gastrointestinal tract, and neural toxicities related to chemotherapy might be significant factors in the development of severe fatigue, and suggested that fatigue could become a dose-limiting factor during chemotherapy. According to the logistic regression analysis, breast cancer patients with no dose reduction were 22.3 times more likely to develop severe fatigue than patients with dose reduction. Wyatt et al. [68] aimed to determine the relationship between chemotherapy interruption and the severity of associated symptoms. They found that the main reason for dose reduction or stoppage was disease progression. In other words, clinical judgment mainly determined the ability of these patients to continue medical therapy, while symptom-related reasons accounted for only 24% of withdrawals [68]. Furthermore, our results showed a statistically significant association between the severity of fatigue and chemotherapy dose delay. Logistic regression indicated that patients who did not experience dose delays were 2.86 times less likely to develop severe fatigue than those who experienced dose delays. This finding contradicts an earlier study that found no association between fatigue severity, chemotherapy dose delay, and dose reduction [68]. Wyatt et al. [68] also reported that pain severity (the main factor associated with fatigue in this study), was a predictor of dose delay or reduction in 385 breast cancer patients. Other studies found that the severity of pain depended on the severity of metastasis, which could indicate to an oncologist that it might be necessary to modify a dose to alleviate a terminal patient’s fatigue levels [69,70].
Overall, the findings of our study suggest that fatigue among breast cancer patients was associated with demographic factors (Malaysian breast cancer survivors who suffered from fatigue were mostly Malay), chemotherapy-related factors (number of regimens, dose delay or dose reduction, receiving docetaxel), and advanced cancer stage. Thus, oncologists and health care professionals should pay particular attention to this specific group of patients. Future research should study additional variables related to fatigue following breast cancer treatment and their impact on QOL over time. Other factors, such as age, menstrual status, employment status, marital status, and type of chemotherapy were not significantly associated with the prevalence of severe CRT.
Conclusion
Fatigue is an important health issue influencing QOL among breast cancer patients. This study provided baseline information on the effect of fatigue on the QOL of Malaysian breast cancer patients. This study also demonstrated that the patients experienced CRF in different ways, and a deeper assessment is therefore needed to identify the main factors associated with fatigue. Appropriate treatments for cancer patients suffering from fatigue are important. The results we obtained showed a strong correlation between QOL and fatigue among breast cancer patients receiving chemotherapy. Since pharmacological medications are used widely in many countries including Malaysia, encouraging clinicians to consider a non-pharmacological management course may play an important role in the treatment outcome and QOL of cancer patients. We hope that these findings will contribute to a better understanding of CRF among cancer patients and facilitate the recognition, evaluation, monitoring, and documentation of prompt treatment.
Strengths of the Study
A major strength of this study is that it involved a moderate sample size of 172 breast cancer patients with no missing data on the fatigue subscales of the FACIT-F and BFI. Since it was conducted at multiple hospitals, the results could be used to standardize and improve clinical practice across multiple cancer treatment centers. Moreover, the patients recruited for the study were at different cancer stages, which provided a heterogeneous study population. This study also used credible questionnaires such as the FACIT-F and BFI scales, which were simple and easy to understand. The time it took to complete the questionnaires was no more than 10 minutes, which was very convenient for all respondents.
Notes
Ethics Approval
The research protocols and procedure of informed consent were approved by research ethics committee of Universiti Teknologi MARA (REC/392/19), Institute Kanser Negara (IKN/500-5/1/25 JId 4 (18), Hospital Kuala Lumpur (HCRC.IIR-2019-07-163), University Malaya Medical Centre & Medical Research Ethical Centre (MREC) (NMRR-18-3902-45218). Researcher adhered to the principles of the Declaration of Helsinki and the Malaysian Good Clinical Practice Guidelines. Participants were briefed about the purpose of the study, and informed consent was obtained from all participants involved in the study.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
None.
Availability of Data
Data and materials are available upon request.
Authors’ Contributions
The author would like to thank all the staff at respective hospitals for their assistance and generosity in providing access to medical records at the clinic.