Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 5(6); 2014 > Article
-
Original Article
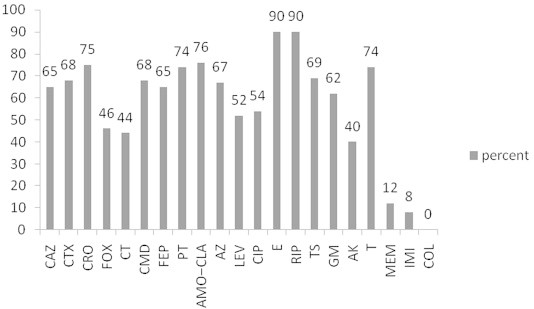
Characterization of Plasmid-Mediated AmpC and Carbapenemases among Iranain Nosocomial Isolates ofKlebsiella pneumoniae Using Phenotyping and Genotyping Methods - Alireza Japoni-Nejada, Ehsanollah Ghaznavi-Radb, Alex van Belkumc,d
-
Osong Public Health and Research Perspectives 2014;5(6):333-338.
DOI: https://doi.org/10.1016/j.phrp.2014.09.003
Published online: November 13, 2014
aDepartment of Mycobacteriology, Pasteur Institute of Iran, Tehran, Iran
bDepartment of Medical Microbiology, Faculty of Medicine, Arak University of Medical Sciences, Arak, Iran
cErasmus MC, Department of Medical Microbiology and Infectious Diseases, Rotterdam, The Netherlands
dbioMérieux, La Balme-les-Grottes, Isère, France
- ∗Corresponding author. alizh63@yahoo.com
• Received: August 6, 2014 • Revised: September 17, 2014 • Accepted: September 23, 2014
© 2014 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the CC-BY-NC License (http://creativecommons.org/licenses/by-nc/3.0).
Figure & Data
References
Citations
Citations to this article as recorded by 

- The Global and Regional Prevalence of Hospital-Acquired Carbapenem-Resistant Klebsiella pneumoniae Infection: A Systematic Review and Meta-analysis
Xing-chen Lin, Chang-li Li, Shao-yang Zhang, Xiao-feng Yang, Meng Jiang
Open Forum Infectious Diseases.2024;[Epub] CrossRef - High frequency of NDM-1 and OXA-48 carbapenemase genes among Klebsiella pneumoniae isolates in central Iran
Elnaz Abbasi, Ehsanollah Ghaznavi-Rad
BMC Microbiology.2023;[Epub] CrossRef - Detection of ESBL and AmpC producing Klebsiella pneumoniae ST11 and ST147 from urinary tract infections in Iran
Shaghayegh Shahkolahi, Pegah Shakibnia, Shahla Shahbazi, Samira Sabzi, Farzad Badmasti, Mohammad Reza Asadi Karam, Mehri Habibi
Acta Microbiologica et Immunologica Hungarica.2022;[Epub] CrossRef - Plasmid-mediated AmpC β-Lactamase gene analysis in Klebsiella Pneumoniae clinical isolates
Nabi Jomehzadeh, Khadijeh Ahmadi, Hasti Shaabaninejad, Gholamali Eslami
Biomedical and Biotechnology Research Journal (BBR.2022; 6(4): 582. CrossRef - Characterization of Antimicrobial Resistance Patterns of Klebsiella pneumoniae Isolates Obtained from Wound Infections
Roya Ghanavati, Hossein Kazemian, Parisa Asadollahi , Hamid Heidari, Gholamreza Irajian, Fatemeh Navab-Moghadam, Shabnam Razavi
Infectious Disorders - Drug Targets .2021; 21(1): 119. CrossRef - An investigation of extended-spectrum β-lactamases (ESBLs) in Klebsiella isolated from foodborne outbreaks in Iran
Farnaz Hajikarim, Mohammad Mehdi Soltan Dallal, Mohammad Reza Pourmand, Milad Abdi
Gene Reports.2020; 19: 100632. CrossRef - Prevalence and Mechanisms of Carbapenem Resistance in Klebsiella pneumoniae and Escherichia coli: A Systematic Review and Meta-Analysis of Cross-Sectional Studies from Iran
Mohammad Javad Nasiri, Mehdi Mirsaeidi, Seyyed Mohammad Javad Mousavi, Mania Arshadi, Fatemeh Fardsanei, Behnaz Deihim, Sara Davoudabadi, Samin Zamani, Bahareh Hajikhani, Hossein Goudarzi, Mehdi Goudarzi, Zahra Sadat Seghatoleslami, Hossein Dabiri, Payam
Microbial Drug Resistance.2020; 26(12): 1491. CrossRef - Characterization of β-lactam resistance in K. pneumoniae associated with ready-to-eat processed meat in Egypt
Shaymaa H. Abdel-Rhman, Grzegorz Woźniakowski
PLOS ONE.2020; 15(9): e0238747. CrossRef - Multiplex PCR to detect pAmpC β-lactamases among enterobacteriaceae at a tertiary care laboratory in Mumbai, India
Mubin Kazi, Kanchan Ajbani, Jeffrey A. Tornheim, Anjali Shetty, Camilla Rodrigues
Microbiology.2019; 165(2): 246. CrossRef - AmpC β lactamases in Urinary Klebsiella pneumoniae Isolates: First Report of ACC Type AmpC β-lactamase Resistance in Iran
Maryam Ghane, Laleh Babaeekhou, Mahdi Jafar Shanjani
Journal of Advances in Medical and Biomedical Rese.2019; 27(123): 23. CrossRef - The Molecular and Clinical Epidemiology of Extended-Spectrum Cephalosporin– and Carbapenem-Resistant Enterobacteriaceae at 4 US Pediatric Hospitals
Danielle M Zerr, Scott J Weissman, Chuan Zhou, Matthew P Kronman, Amanda L Adler, Jessica E Berry, Jaipreet Rayar, Jeff Myers, Wren L Haaland, Carey-Ann D Burnham, Alexis Elward, Jason Newland, Rangaraj Selvarangan, Kaede V Sullivan, Theoklis Zaoutis, Xua
Journal of the Pediatric Infectious Diseases Socie.2017; 6(4): 366. CrossRef - Previous Antibiotic Exposure Increases Risk of Infection with Extended-Spectrum-β-Lactamase- and AmpC-Producing Escherichia coli and Klebsiella pneumoniae in Pediatric Patients
Danielle M. Zerr, Arianna Miles-Jay, Matthew P. Kronman, Chuan Zhou, Amanda L. Adler, Wren Haaland, Scott J. Weissman, Alexis Elward, Jason G. Newland, Theoklis Zaoutis, Xuan Qin
Antimicrobial Agents and Chemotherapy.2016; 60(7): 4237. CrossRef - Molecular characterization of extended-spectrum β-lactamase, plasmid-mediated AmpC cephalosporinase and carbapenemase genes among Enterobacteriaceae isolates in five medical centres of East and West Azerbaijan, Iran
Mohammad Reza Sadeghi, Reza Ghotaslou, Mohammad Taghi Akhi, Mohammad Asgharzadeh, Alka Hasani
Journal of Medical Microbiology .2016; 65(11): 1322. CrossRef - High Prevalence of AmpC β-Lactamases in Clinical Isolates of Escherichia coli in Ilam, Iran
Abbas Maleki, Afra Khosravi, Sobhan Ghafourian, Iraj Pakzad, Shiva Hosseini, Rashid Ramazanzadeh, Nourkhoda Sadeghifard
Osong Public Health and Research Perspectives.2015; 6(3): 201. CrossRef



 PubReader
PubReader Cite
Cite
