Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 5(3); 2014 > Article
-
Original Article
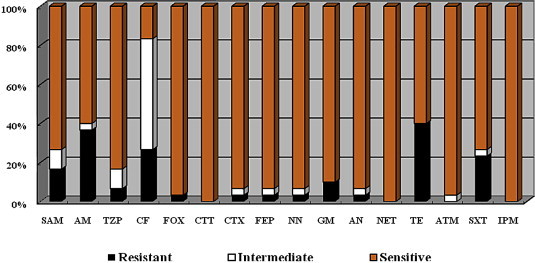
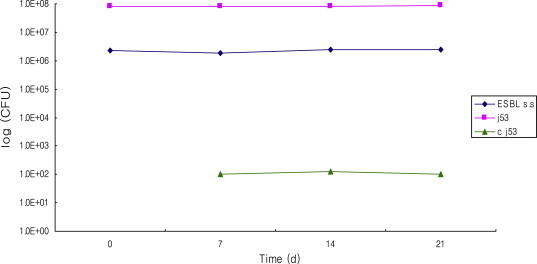
Possibility of CTX-M-14 Gene Transfer fromShigella sonnei to a CommensalEscherichia coli Strain of the Gastroenteritis Microbiome - Seung-Hak Cho, Soon Young Han, Yeon-Ho Kang
-
Osong Public Health and Research Perspectives 2014;5(3):156-160.
DOI: https://doi.org/10.1016/j.phrp.2014.04.007
Published online: May 14, 2014
Division of Enteric Bacterial Infections, Korea National Institute of Health, Osong, Korea
- ∗Corresponding author. skcho38@korea.kr
• Received: April 2, 2014 • Revised: April 24, 2014 • Accepted: April 27, 2014
© 2014 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Figure & Data
References
Citations
Citations to this article as recorded by 

- Antibiotics in avian care and husbandry-status and alternative antimicrobials
Adam Lepczyński, Agnieszka Herosimczyk, Mateusz Bucław, Michalina Adaszyńska-Skwirzyńska
Physical Sciences Reviews.2024; 9(2): 701. CrossRef - Healthy broilers disseminate antibiotic resistance in response to tetracycline input in feed concentrates
S. Sreejith, Shamna Shajahan, P.R. Prathiush, V.M. Anjana, Arathy Viswanathan, Vishnu Chandran, G.S. Ajith Kumar, R. Jayachandran, Jyothis Mathew, E.K. Radhakrishnan
Microbial Pathogenesis.2020; 149: 104562. CrossRef - Molecular Characterization of Resistance Genes in MDR-ESKAPE Pathogens
Masoumeh Navidinia, Mehdi Goudarzi, Samira Molaei Rameshe, Zahra Farajollahi, Pedram Ebadi Asl, Saeed Zaka khosravi, Mohammad Reza Mounesi
Journal of Pure and Applied Microbiology.2017; 11(2): 779. CrossRef


 PubReader
PubReader Cite
Cite
