Search
- Page Path
- HOME > Search
Special Article
- Challenges in capacity building of national immunization programs and emergency or pandemic vaccination responses in the Global Health Security Agenda member countries
- Sookhyun Lee, Jung Ju Oh, Sang Hyun Park, Dasol Ro, Ye Jin Jeong, So Yoon Kim
- Received June 14, 2023 Accepted January 16, 2024 Published online March 28, 2024
- DOI: https://doi.org/10.24171/j.phrp.2023.0159 [Epub ahead of print]
- 181 View
- 9 Download
-
 Abstract
Abstract
 PDF
PDF - The Immunization Action Package aims to increase the vaccination rate for vaccine-preventable diseases to save lives. To achieve this, member countries of the Global Health Security Agenda (GHSA) must have the capacity to implement sustainable national immunization programs (NIPs) and to respond to emergency vaccination scenarios. This article focuses on 4 major areas of NIP capacity, including in emergency situations: infrastructure capacity, sustainable financing capacity, vaccine access and equity, and vaccination hesitancy. Countries require resilient infrastructure to achieve high vaccination rates and develop preparedness for public health emergencies. Financial sustainability is crucial in achieving high vaccination coverage to best implement initiatives and national programs. Furthermore, challenges to NIPs include vaccine access and equity, as inequitable distribution and access to vaccines for coronavirus disease 2019 accelerated the impact of the pandemic. Lastly, the correlation between low acceptance and successful implementation of national initiatives suggests that vaccination hesitancy is another challenge to NIPs. In an attempt to overcome these challenges, the Expert Forum of the GHSA Seventh Ministerial Meeting was held to provide sessions allowing countries to share their national case studies and discuss strategies for capacity building of country-level NIPs, including for emergency responses.
Original Articles
- Prevalence and patterns of adverse events following childhood immunization and the responses of mothers in Ile-Ife, South West Nigeria: a facility-based cross-sectional survey
- Olorunfemi Akinbode Ogundele, Funmito Omolola Fehintola, Mubarak Salami, Rahmat Usidebhofoh, Mary Aderemi Abaekere
- Osong Public Health Res Perspect. 2023;14(4):291-299. Published online July 27, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0071
- 2,189 View
- 133 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 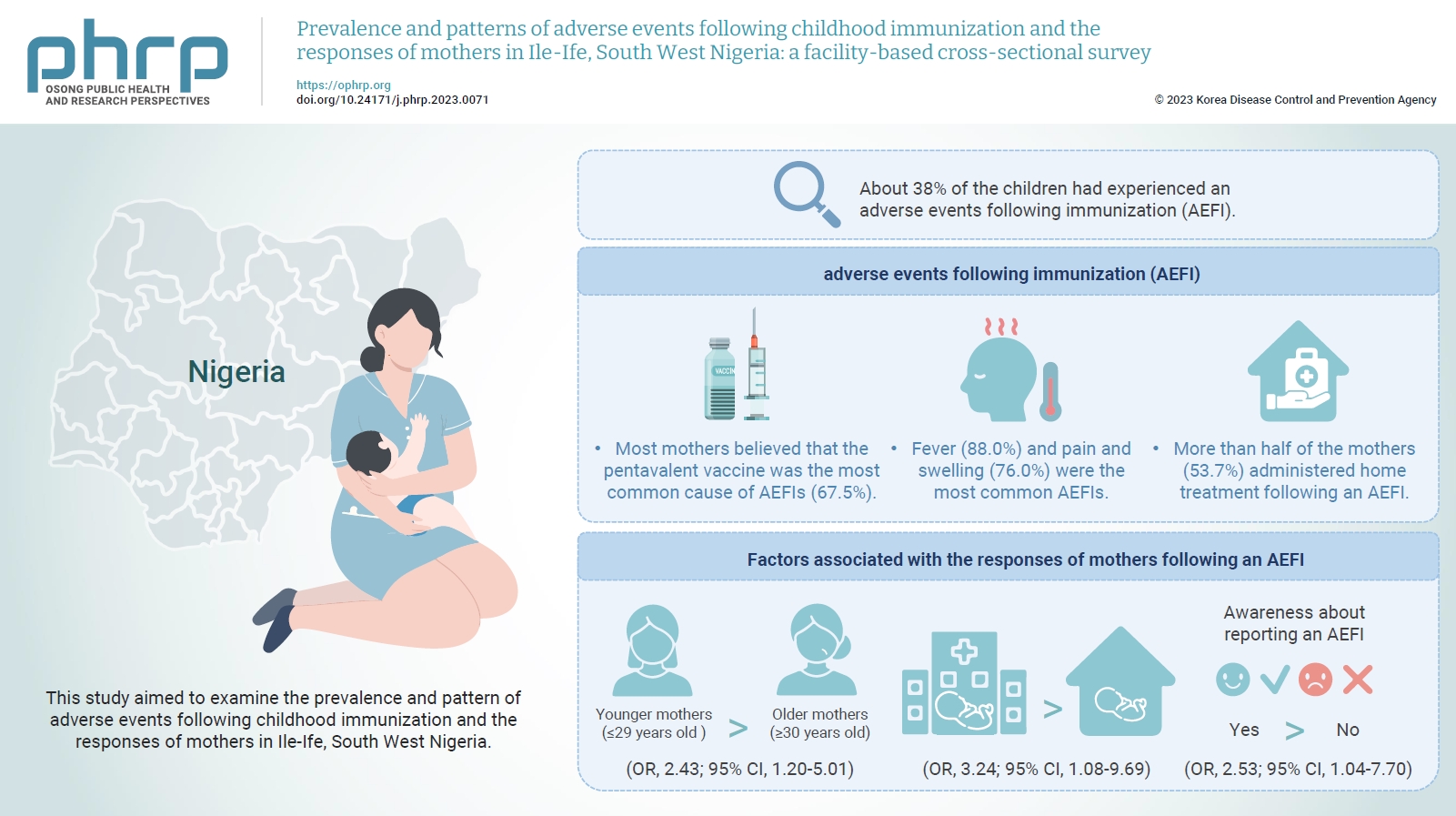
- Objectives
This study aimed to examine the prevalence and pattern of adverse events following childhood immunization and the responses of mothers in Ile-Ife, South West Nigeria.
Methods
This descriptive cross-sectional study was conducted among 422 mothers of children aged 0 to 24 months attending any of the 3 leading immunization clinics in Ile-Ife, Nigeria. The respondents were selected using the multi-stage sampling technique. Data were collected using a pretested structured interviewer-administered questionnaire and analyzed using IBM SPSS ver. 26.0. The chi-square test was used to test associations, while binary logistic regression was used to determine the predictors of mothers’ responses to adverse events following immunization (AEFIs). A p-value of <0.05 was considered statistically significant.
Results
The mean age of the respondents was 29.99±5.74 years. About 38% of the children had experienced an AEFI. Most mothers believed that the pentavalent vaccine was the most common cause of AEFIs (67.5%). Fever (88.0%) and pain and swelling (76.0%) were the most common AEFIs. More than half of the mothers (53.7%) administered home treatment following an AEFI. Younger mothers (odds ratio [OR], 2.43; 95% confidence interval [CI], 1.20–5.01), mothers who delivered their children at a healthcare facility (OR, 3.24; 95% CI, 1.08–9.69), and mothers who were knowledgeable about reporting AEFIs (OR, 2.53; 95% CI, 1.04–7.70) were most likely to respond appropriately to AEFIs.
Conclusion
The proportion of mothers who responded poorly to AEFIs experienced by their children was significant. Therefore, strategies should be implemented to improve mothers’ knowledge about AEFIs to improve their responses.
- The incidence and clinical characteristics of myocarditis and pericarditis following mRNA-based COVID-19 vaccination in Republic of Korea adolescents from July 2021 to September 2022
- Ju-Young Sim, Seung-Yun Kim, Eun-Kyoung Kim
- Osong Public Health Res Perspect. 2023;14(2):76-88. Published online April 18, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0032
- 3,876 View
- 231 Download
- 2 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 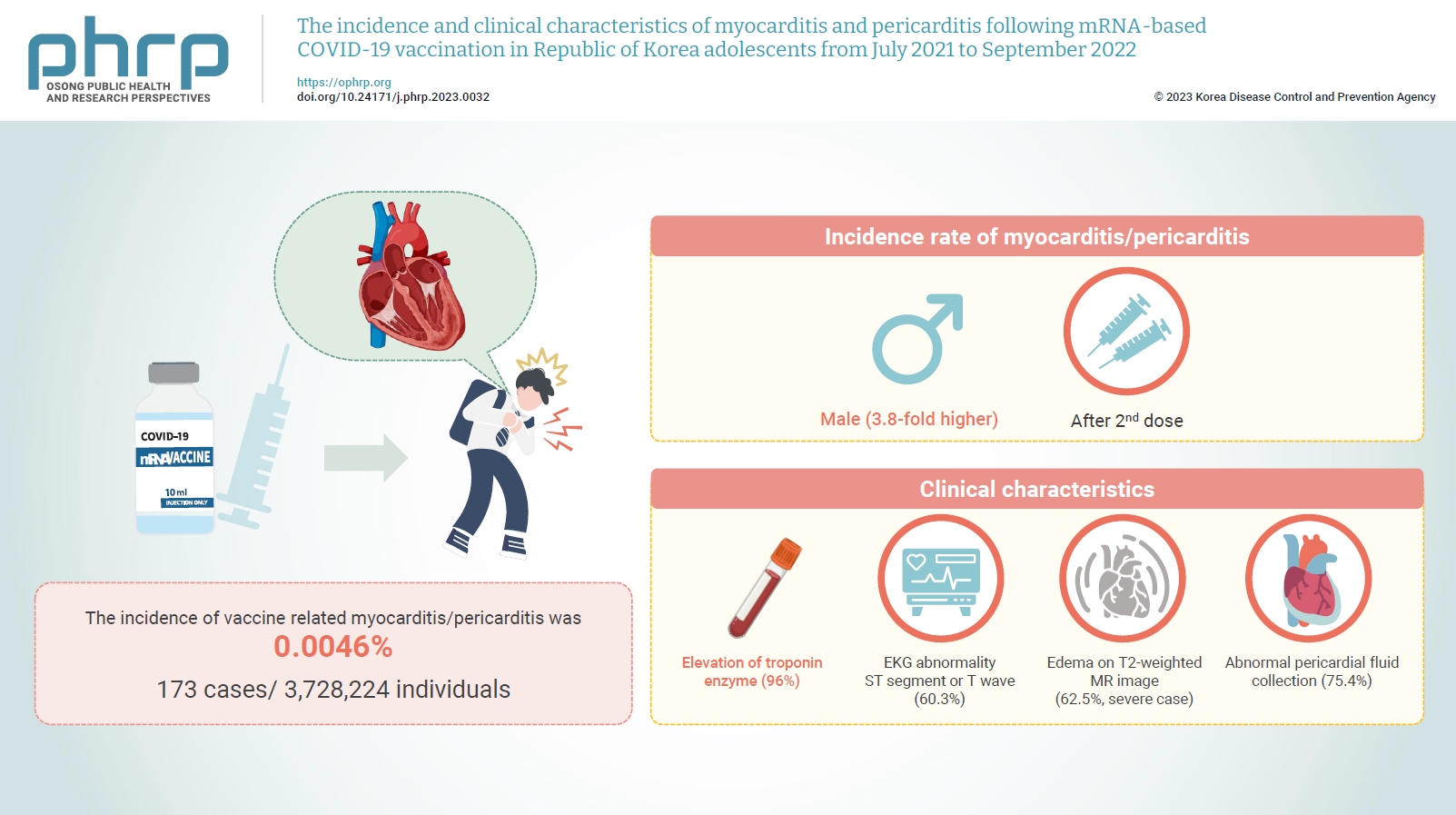
- Objectives
Age-specific information regarding myocarditis/pericarditis in adolescents following mRNA-based coronavirus disease 2019 (COVID-19) vaccination in Asia remains insufficient. This study investigated the incidence and clinical characteristics of myocarditis/pericarditis in Republic of Korea adolescents after mRNA-based COVID-19 vaccination.
Methods
This retrospective descriptive study utilized patient data from the Korea Immunization Management System. Incidence rates were calculated according to age and sex. Clinical characteristics (symptoms/signs, laboratory values, and imaging results) were compared between mild and severe cases.
Results
Between July 19, 2021 and September 30, 2022, 3,728,224 individuals aged 12 to 19 years received 6,484,165 mRNA-based COVID-19 vaccines, and 173 cases met the case definition for myocarditis/pericarditis: 151 mild (87.3%) and 22 severe (12.7%). The incidence was 3.8-fold higher in males than in females. Troponin I/ troponin T was elevated in 96% of myocarditis cases, demonstrating higher sensitivity than creatine kinase-myocardial band (67.6%) or C-reactive protein (75.2%). ST-segment or Twave on electrography abnormalities were found in 60.3% (85/141). Paroxysmal/sustained atrial/ventricular arrhythmias were more common in severe than in mild cases (45.5% vs. 16.8%, p=0.008). Edema on T2-weighted magnetic imaging occurred in 21.6% (8/37) and 62.5% (5/8) of mild and severe cases, respectively (p=0.03). Abnormal pericardial fluid collection or pericardial inflammation was found in 75.4% of pericarditis cases (49/65).
Conclusion
Myocarditis/pericarditis occurred in rare cases following mRNA-based COVID-19 vaccination. Most cases were mild, but the incidence was higher in adolescent males and after the second dose. As bivalent severe acute respiratory syndrome coronavirus 2 mRNA vaccination started in Republic of Korea in October 2022, the post-vaccination incidence of myocarditis/pericarditis should be closely monitored, considering clinical characteristics. -
Citations
Citations to this article as recorded by- Responses to Common Misconceptions Relating to COVID-19 Variant-Adapted mRNA Vaccines
George Kassianos, Pauline MacDonald, Ivan Aloysius, Shanti Pather
Vaccines.2024; 12(1): 57. CrossRef - To become a more stronger and safer country
Jong-Koo Lee
Osong Public Health and Research Perspectives.2023; 14(2): 67. CrossRef
- Responses to Common Misconceptions Relating to COVID-19 Variant-Adapted mRNA Vaccines
- Estimated impact of the national hepatitis B immunization program on acute viral hepatitis B among adolescents in Republic of Korea
- Chungman Chae, Sangwoo Tak
- Osong Public Health Res Perspect. 2023;14(2):138-145. Published online March 24, 2023
- DOI: https://doi.org/10.24171/j.phrp.2022.0321
- 1,496 View
- 62 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 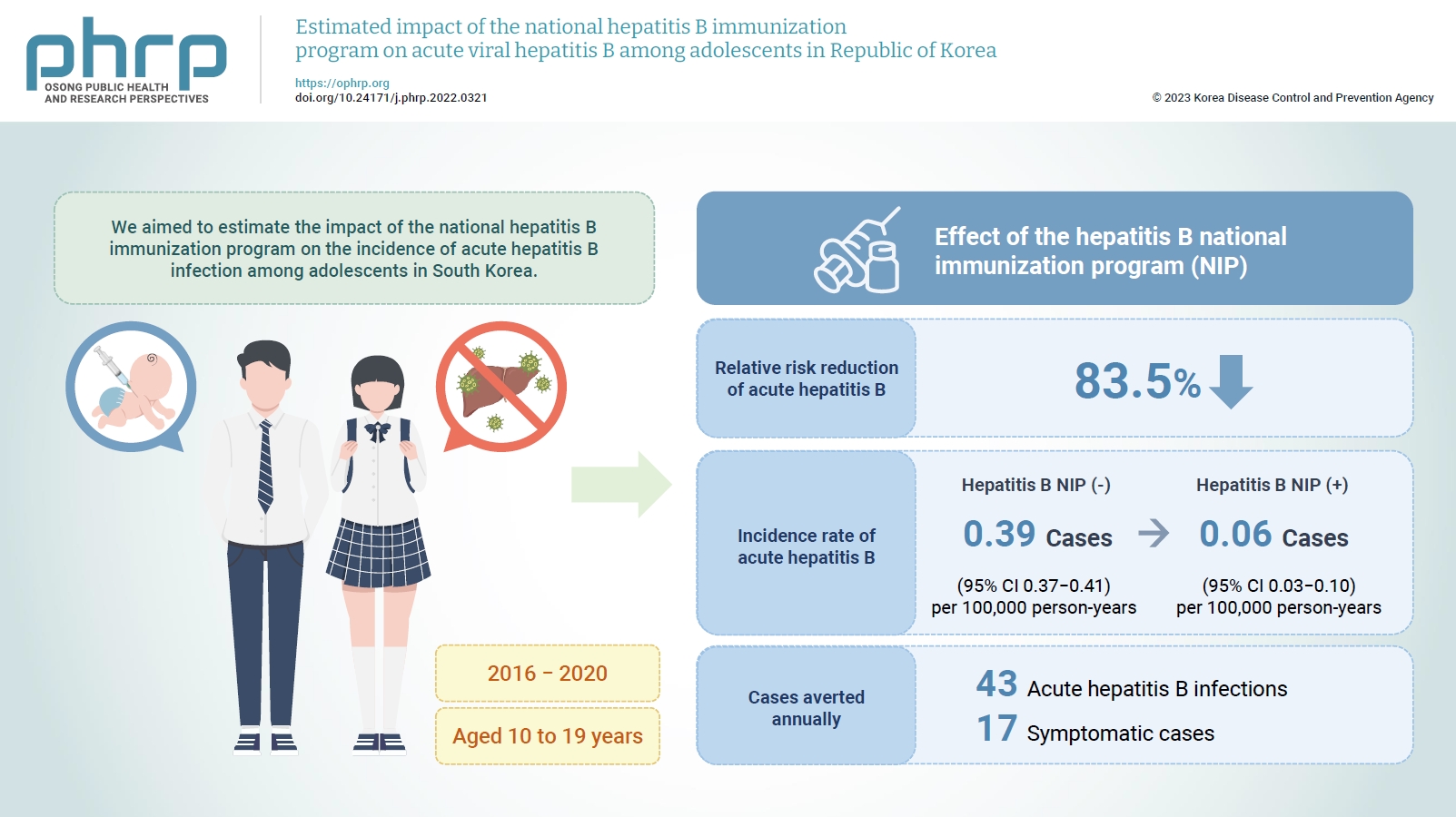
- Objectives
We aimed to estimate the impact of the national hepatitis B immunization program on the incidence of acute hepatitis B infection among adolescents in South Korea.
Methods
We estimated the counterfactual incidence rate of reported acute hepatitis B among adolescents from 2016 to 2020 compared to the assumption that the national hepatitis B immunization program for children had not been implemented since 1995. The impact of the national hepatitis B immunization program for adolescents was measured by estimating the absolute risk reduction and averted acute hepatitis B infections among adolescents from 2016 to 2020 attributed to the national immunization program.
Results
The relative risk reduction of acute hepatitis B among adolescents was estimated to be 83.5% after implementing the national hepatitis B immunization program. The incidence rate of reported acute hepatitis B infections among adolescents decreased from 0.39 to 0.06 per 100,000 person-years, and 43 acute hepatitis B infections, including 17 symptomatic cases, were averted annually from 2016 to 2020 by the national hepatitis B immunization program.
Conclusion
The national hepatitis B immunization program for children was effective in preventing acute hepatitis B infection among adolescents in South Korea.
- COVID-19 outbreak response at a nursing hospital in South Korea in the post-vaccination era, including an estimation of the effectiveness of the first shot of the Oxford-AstraZeneca COVID-19 vaccine (ChAdOx1-S)
- Chanhee Kim, Geon Kang, Sun Gu Kang, Heeyoung Lee
- Osong Public Health Res Perspect. 2022;13(2):114-122. Published online April 26, 2022
- DOI: https://doi.org/10.24171/j.phrp.2021.0262
- 4,140 View
- 92 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Objectives
We descriptively reviewed a coronavirus disease 2019 (COVID-19) outbreak at a nursing hospital in Gyeonggi Province (South Korea) and assessed the effectiveness of the first dose of the Oxford-AstraZeneca vaccine in a real-world population. Methods: The general process of the epidemiological investigation included a public health intervention. The relative risk (RR) of vaccinated and unvaccinated groups was calculated and compared to confirm the risk of severe acute respiratory syndrome coronavirus-2 (SARSCoV-2) infection, and vaccine effectiveness was evaluated based on the calculated RR. Results: The population at risk was confined to ward E among 8 wards of Hospital X, where the outbreak occurred. This population comprised 55 people, including 39 patients, 12 nurses, and 4 caregivers, and 19 cases were identified. The RR between the vaccinated and unvaccinated groups was 0.04, resulting in a vaccine effectiveness of 95.3%. The vaccination rate of the nonpatients in ward E was the lowest in the entire hospital, whereas the overall vaccination rate of the combined patient and non-patient groups in ward E was the third lowest. Conclusion: The first dose of the Oxford-AstraZeneca vaccine (ChAdOx1-S) was effective in preventing SARS-CoV-2 infection. To prevent COVID-19 outbreaks in medical facilities, it is important to prioritize the vaccination of healthcare providers. -
Citations
Citations to this article as recorded by- COVID-19 Vaccination in Korea: Past, Present, and the Way Forward
Eliel Nham, Joon Young Song, Ji Yun Noh, Hee Jin Cheong, Woo Joo Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef
- COVID-19 Vaccination in Korea: Past, Present, and the Way Forward
- Factors Related to Completed Status and Seropositivity of Hepatitis A Immunization Among Children Aged 1–3 Years and 6–8 Years in South Korea
- Jee-Young Hong, Mo Ran Ki, Hye-Jung Hwang, Delacroix Sinny, Young-Joon Park, Geun-Ryang Bae, Moo-Sik Lee
- Osong Public Health Res Perspect. 2013;4(2):93-98. Published online April 30, 2013
- DOI: https://doi.org/10.1016/j.phrp.2013.02.004
- 3,559 View
- 11 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - This study was designed to identify factors associated with hepatitis A immunization status and seropositivity in Korean children. In-person interviews, reviewing their vaccination cards and testing hepatitis A antibody were conducted with 389 children aged 1–3 years and 544 children aged 6–8 years. In all age groups, earlier birth order was the only significant factor in children receiving either single or both doses of the vaccination. And completion of the second dose of vaccination was a prerequisite for increased seropositivity. Additionally, household income had a positive impact on seropositivity only in children aged 6–8 years. Our findings suggest that presence of an economic barrier is the underlying cause of the decreased hepatitis A vaccination services in Korea. Therefore, hepatitis A vaccine should be included in the essential National Immunization Program.
-
Citations
Citations to this article as recorded by- Vaccine Uptake to Prevent Meningitis and Encephalitis in Shanghai, China
Hairenguli Maimaiti, Jia Lu, Xiang Guo, Lu Zhou, Linjie Hu, Yihan Lu
Vaccines.2022; 10(12): 2054. CrossRef - Changing sero-epidemiology of hepatitis A in Asia Pacific countries: A systematic review
Marissa Gripenberg, Naveena Aloysia D’Cor, Maïna L’Azou, Grenville Marsh, Sophie Druelles, Joshua Nealon
International Journal of Infectious Diseases.2018; 68: 13. CrossRef
- Vaccine Uptake to Prevent Meningitis and Encephalitis in Shanghai, China



 First
First Prev
Prev


