Previous issues
- Page Path
- HOME > Articles and issues > Previous issues
Editorial
- How to deal with the Delta variant this fall
- Jong-Koo Lee
- Osong Public Health Res Perspect. 2021;12(4):201-202. Published online August 26, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0217
- 4,170 View
- 72 Download
- 5 Web of Science
- 5 Crossref
-
 PDF
PDF -
Citations
Citations to this article as recorded by- A Possible Type IV Hypersensitivity Reaction to Older Antiepileptic Drugs During and
After Recovery from COVID-19 Infection
Mohsen Khosravi
Pharmacopsychiatry.2022; 55(01): 58. CrossRef - Points to consider for COVID-19 vaccine quality control and national lot release in Republic of Korea: focus on a viral vector platform
Jung Hun Ju, Naery Lee, Sun-hee Kim, Seokkee Chang, Misook Yang, Jihyun Shin, Eunjo Lee, Sunhwa Sung, Jung-Hwan Kim, Jin Tae Hong, Ho Jung Oh
Osong Public Health and Research Perspectives.2022; 13(1): 4. CrossRef - Broad humoral and cellular immunity elicited by one-dose mRNA vaccination 18 months after SARS-CoV-2 infection
Chang Kyung Kang, Hyun Mu Shin, Pyoeng Gyun Choe, Jiyoung Park, Jisu Hong, Jung Seon Seo, Yung Hie Lee, Euijin Chang, Nam Joong Kim, Minji Kim, Yong-Woo Kim, Hang-Rae Kim, Chang-Han Lee, Jun-Young Seo, Wan Beom Park, Myoung-don Oh
BMC Medicine.2022;[Epub] CrossRef - Impact of national Covid-19 vaccination Campaign, South Korea
Seonju Yi, Young June Choe, Do Sang Lim, Hye Roen Lee, Jia Kim, Yoo-Yeon Kim, Ryu Kyung Kim, Eun Jung Jang, Sangwon Lee, Eunjoo Park, Seung-Jin Kim, Young-Joon Park
Vaccine.2022; 40(26): 3670. CrossRef - Recent increase in the detection of human parainfluenza virus during the coronavirus disease-2019 pandemic in the Republic of Korea
Heui Man Kim, Jee Eun Rhee, Nam-Joo Lee, Sang Hee Woo, Ae Kyung Park, Jaehee Lee, Cheon Kwon Yoo, Eun-Jin Kim
Virology Journal.2022;[Epub] CrossRef
- A Possible Type IV Hypersensitivity Reaction to Older Antiepileptic Drugs During and
After Recovery from COVID-19 Infection
Review Articles
- Scrutiny of COVID-19 response strategies among severely affected European nations
- Shine Stephen, Alwin Issac, Rakesh Vadakkethil Radhakrishnan, Jaison Jacob, VR Vijay, Sam Jose, SM Azhar, Anoop S. Nair, Nadiya Krishnan, Rakesh Sharma, Manju Dhandapani
- Osong Public Health Res Perspect. 2021;12(4):203-214. Published online July 29, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0068
- 10,393 View
- 128 Download
- 2 Web of Science
- 7 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 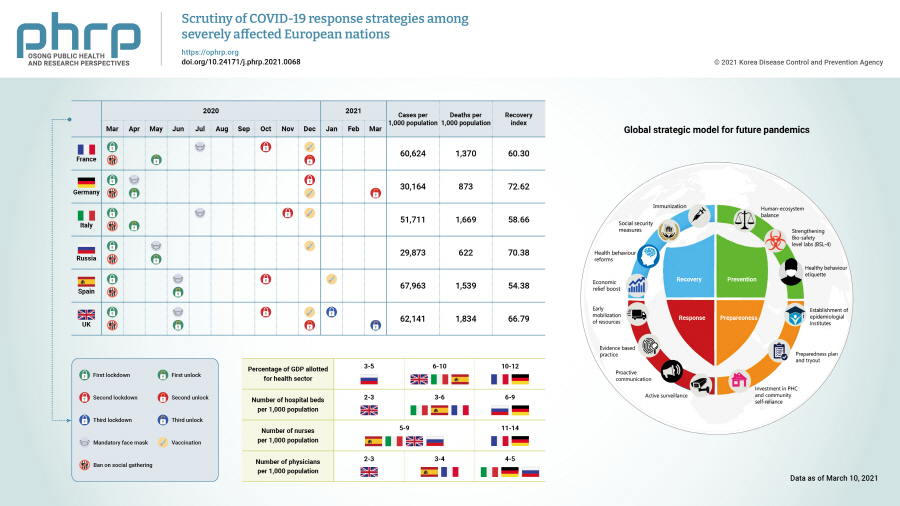
- Although the health care systems in Europe are considered the global benchmark, European nations were severely affected by the coronavirus disease 2019 (COVID-19) pandemic. This manuscript aimed to examine the strategies implemented to combat the COVID-19 pandemic by France, the United Kingdom, Spain, Italy, Germany, and Russia and their outcomes in terms of the number of cases, testing, and deaths. This is the first review of its kind that extensively analyzes the preparedness, mitigation, and response strategies against the COVID-19 pandemic adopted by these nations. This paper further suggests a strategic preparedness model for future pandemics. From the analysis, we found that a decentralized approach, prompt decision-making and timely execution, coordination between local health authorities, and public participation in the implementation of strategies could substantially reduce the case fatality rate. Nations with a high percentage of gross domestic product invested in the health sector, as well as more nurses, physicians, hospital beds, intensive care unit beds, and ventilators, better managed the pandemic. Instead, nations that postponed their pandemic response by delaying tracking, tracing, testing, quarantine, and lockdown were badly affected. The lessons learned from the present pandemic could be used as a guide to prepare for further pandemics.
-
Citations
Citations to this article as recorded by- Psychological impact and development of autistic traits in children during the COVID 19 Pandemic: A study through Guardian
Waseem Iqbal, Mudassir Hassan, Parveez Ahmed Mir, Syed Kaiser
IP International Journal of Medical Paediatrics an.2024; 9(4): 135. CrossRef - The Safety of Covishield (ChAdOx1 nCoV-19) Vaccine after Three Doses Vaccination under Mass Vaccination Program of Government of India
Narinder Singh, Jaswinder Singh, Vikram Bhandari, Rahat Kumar
AMEI's Current Trends in Diagnosis & Treatment.2024; 7(2): 38. CrossRef - Antibody titre in infants of covid-19 infected mothers
Shivani Sharma, Pushkar Lal Meena, Rameshwar Lal Suman, Jaya Ninama
IP International Journal of Medical Paediatrics an.2023; 9(2): 68. CrossRef - A study to assess the level of stress among nursing students of IUST during COVID-19 pandemic
Javaid Ahmad Mir, Asmat Parveen, Suheel Rashid Wani, Tayyibah Nisar, Sakeena Majeed, Wahida Kausar, Basit Ul Islam
IP International Journal of Medical Paediatrics an.2022; 8(1): 15. CrossRef - Strategy to prevent infection from Covid-19 among security officers of tertiary care centre: A preexperimental study
Rakesh Sharma, KusumK Rohilla, Lisa Chadha, Priyanka Malhotra, S Sharmila, Prasuna Jelly
Journal of Family Medicine and Primary Care.2021; 10(9): 3257. CrossRef - Post COVID-19 changes in the perception of the parents towards dentistry for their child
Nahid Iftikhar, Shalini Dixit, Aditi Yadav
IP International Journal of Medical Paediatrics an.2021; 7(3): 155. CrossRef - A comparative study of attitudes towards COVID-19 vaccination in the rural and urban population of Uttarakhand, India
Rakesh Sharma, Prasuna Jelly, Vishwas AS, Lisa Chadha, Vartika Saxena, Latika Mohan
Journal of Global Health Economics and Policy.2021;[Epub] CrossRef
- Psychological impact and development of autistic traits in children during the COVID 19 Pandemic: A study through Guardian
- COVID-19 prediction models: a systematic literature review
- Sheikh Muzaffar Shakeel, Nithya Sathya Kumar, Pranita Pandurang Madalli, Rashmi Srinivasaiah, Devappa Renuka Swamy
- Osong Public Health Res Perspect. 2021;12(4):215-229. Published online August 13, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0100
- 8,265 View
- 185 Download
- 15 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF - As the world grapples with the problem of the coronavirus disease 2019 (COVID-19) pandemic and its devastating effects, scientific groups are working towards solutions to mitigate the effects of the virus. This paper aimed to collate information on COVID-19 prediction models. A systematic literature review is reported, based on a manual search of 1,196 papers published from January to December 2020. Various databases such as Google Scholar, Web of Science, and Scopus were searched. The search strategy was formulated and refined in terms of subject keywords, geographical purview, and time period according to a predefined protocol. Visualizations were created to present the data trends according to different parameters. The results of this systematic literature review show that the study findings are critically relevant for both healthcare managers and prediction model developers. Healthcare managers can choose the best prediction model output for their organization or process management. Meanwhile, prediction model developers and managers can identify the lacunae in their models and improve their data-driven approaches.
-
Citations
Citations to this article as recorded by- The Telemedicine Demand Index and its Utility in Managing COVID-19 Case Surges
Martin Yong Kwong Lee, Kie Beng Goh, Deanna Xiuting Koh, Si Jack Chong, Raymond Swee Boon Chua
Telemedicine and e-Health.2024; 30(2): 545. CrossRef - Vaccination compartmental epidemiological models for the delta and omicron SARS-CoV-2 variants
J. Cuevas-Maraver, P.G. Kevrekidis, Q.Y. Chen, G.A. Kevrekidis, Y. Drossinos
Mathematical Biosciences.2024; 367: 109109. CrossRef - The reporting completeness and transparency of systematic reviews of prognostic prediction models for COVID-19 was poor: a methodological overview of systematic reviews
Persefoni Talimtzi, Antonios Ntolkeras, Georgios Kostopoulos, Konstantinos I. Bougioukas, Eirini Pagkalidou, Andreas Ouranidis, Athanasia Pataka, Anna-Bettina Haidich
Journal of Clinical Epidemiology.2024; 167: 111264. CrossRef - A comprehensive benchmark for COVID-19 predictive modeling using electronic health records in intensive care
Junyi Gao, Yinghao Zhu, Wenqing Wang, Zixiang Wang, Guiying Dong, Wen Tang, Hao Wang, Yasha Wang, Ewen M. Harrison, Liantao Ma
Patterns.2024; 5(4): 100951. CrossRef - A study of learning models for COVID-19 disease prediction
Sakshi Jain, Pradeep Kumar Roy
Journal of Ambient Intelligence and Humanized Comp.2024; 15(4): 2581. CrossRef - Is It Possible to Predict COVID-19? Stochastic System Dynamic Model of Infection Spread in Kazakhstan
Berik Koichubekov, Aliya Takuadina, Ilya Korshukov, Anar Turmukhambetova, Marina Sorokina
Healthcare.2023; 11(5): 752. CrossRef - Early triage echocardiography to predict outcomes in patients admitted with COVID‐19: a multicenter study
Daniel Peck, Andrea Beaton, Maria Carmo Nunes, Nicholas Ollberding, Allison Hays, Pranoti Hiremath, Federico Asch, Nitin Malik, Christopher Fung, Craig Sable, Bruno Nascimento
Echocardiography.2023; 40(5): 388. CrossRef - Static Seeding and Clustering of LSTM Embeddings to Learn From Loosely Time-Decoupled Events
Christian G. Manasseh, Razvan Veliche, Jared Bennett, Hamilton Scott Clouse
IEEE Access.2023; 11: 64219. CrossRef - Harnessing the power of AI: Advanced deep learning models optimization for accurate SARS-CoV-2 forecasting
Muhammad Usman Tariq, Shuhaida Binti Ismail, Muhammad Babar, Ashir Ahmad, Lin Wang
PLOS ONE.2023; 18(7): e0287755. CrossRef - Development and validation of COEWS (COVID-19 Early Warning Score) for hospitalized COVID-19 with laboratory features: A multicontinental retrospective study
Riku Klén, Ivan A Huespe, Felipe Aníbal Gregalio, Antonio Lalueza Lalueza Blanco, Miguel Pedrera Jimenez, Noelia Garcia Barrio, Pascual Ruben Valdez, Matias A Mirofsky, Bruno Boietti, Ricardo Gómez-Huelgas, José Manuel Casas-Rojo, Juan Miguel Antón-Santos
eLife.2023;[Epub] CrossRef - Dynamic transmission modeling of COVID-19 to support decision-making in Brazil: A scoping review in the pre-vaccine era
Gabriel Berg de Almeida, Lorena Mendes Simon, Ângela Maria Bagattini, Michelle Quarti Machado da Rosa, Marcelo Eduardo Borges, José Alexandre Felizola Diniz Filho, Ricardo de Souza Kuchenbecker, Roberto André Kraenkel, Cláudia Pio Ferreira, Suzi Alves Cam
PLOS Global Public Health.2023; 3(12): e0002679. CrossRef - Predictive Models for Forecasting Public Health Scenarios: Practical Experiences Applied during the First Wave of the COVID-19 Pandemic
Jose M. Martin-Moreno, Antoni Alegre-Martinez, Victor Martin-Gorgojo, Jose Luis Alfonso-Sanchez, Ferran Torres, Vicente Pallares-Carratala
International Journal of Environmental Research an.2022; 19(9): 5546. CrossRef - Artificial intelligence and clinical deterioration
James Malycha, Stephen Bacchi, Oliver Redfern
Current Opinion in Critical Care.2022; 28(3): 315. CrossRef
- The Telemedicine Demand Index and its Utility in Managing COVID-19 Case Surges
Original Articles
- The current status of sexually transmitted infections in South Korean children in the last 10 years
- Yumi Jang, Eunjung Oh
- Osong Public Health Res Perspect. 2021;12(4):230-235. Published online August 4, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0046
- 5,270 View
- 114 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Objectives
This study aimed to determine the status of sexually transmitted infections (STIs) in children in South Korea between 2010 and 2019), as well as to establish preventive maintenance guidelines to reduce the incidence of STIs in children.
Methods
Data reports from 590 STI surveillance systems in community clinics, hospital-level medical institutions with urology or obstetrics/gynecology departments and public hospitals between 2010 and 2019 in the integrative disease management system of the Korea Disease Control and Prevention Agency as of December 2020 were analyzed.
Results
A total of 172,645 cases of STIs were reported over the 10-year period (2010–2019), of which 2,179 cases (1.26%) represented STIs in children below the age of 18 years. A higher incidence of infections was observed in girls (1,499 cases, 68.79%) than in boys (680 cases, 31.21%). The STIs that had the highest incidence were, in descending order, chlamydia (997 cases, 45.75%), gonorrhea (592 cases, 27.17%), genital warts (338 cases, 15.51%), genital herpes (250 cases, 11.47%), and chancroid (2 cases, 0.09%). In adolescents aged 14 to 17 years, chlamydia, genital herpes, and gonorrhea were most frequently reported. Genital warts, in particular, have been consistently reported in children below the age of 14 years.
Conclusion
Children must be protected legally and institutionally from sexual abuse. Specific management protocols for STIs in children must be established by local governments and associated organizations. National human papillomavirus vaccination programs should be expanded to include boys, and anti-STI educational efforts using modern media should be implemented.
- Perceptions of the COVID-19 vaccine and willingness to receive vaccination among health workers in Nigeria
- Oluseyi Ademola Adejumo, Olorunfemi Akinbode Ogundele, Cynthia Roli Madubuko, Rosena Olubanke Oluwafemi, Ogochukwu Chinedum Okoye, Kenechukwu Chukwuemeka Okonkwo, Sunday Samson Owolade, Oladimeji Adedeji Junaid, Olutoyin Morenike Lawal, Adenike Christianah Enikuomehin, Maureen Iru Ntaji, Aisha Sokunbi, Aina Omodele Timothy, Olatunji Sunday Abolarin, Emmanuel Olalekan Ali, John Oghenevwirhe Ohaju-Obodo
- Osong Public Health Res Perspect. 2021;12(4):236-243. Published online July 19, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0023
- 11,525 View
- 455 Download
- 39 Web of Science
- 36 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Objectives
The study aimed to examine health workers’ perceptions of the coronavirus disease 2019 (COVID-19) vaccine in Nigeria and their willingness to receive the vaccine when it becomes available.
Methods
This multi-center cross-sectional study used non-probability convenience sampling to enroll 1,470 hospital workers aged 18 and above from 4 specialized hospitals. A structured and validated self-administered questionnaire was used for data collection. Data entry and analysis were conducted using IBM SPSS ver. 22.0.
Results
The mean age of respondents was 40±6 years. Only 53.5% of the health workers had positive perceptions of the COVID-19 vaccine, and only slightly more than half (55.5%) were willing to receive vaccination. Predictors of willingness to receive the COVID-19 vaccine included having a positive perception of the vaccine (adjusted odds ratio [AOR], 4.55; 95% confidence interval [CI], 3.50−5.69), perceiving a risk of contracting COVID-19 (AOR, 1.50; 95% CI, 1.25–3.98), having received tertiary education (AOR, 3.50; 95% CI, 1.40−6.86), and being a clinical health worker (AOR, 1.25; 95% CI, 1.01−1.68).
Conclusion
Perceptions of the COVID-19 vaccine and willingness to receive the vaccine were sub-optimal among this group. Educational interventions to improve health workers' perceptions and attitudes toward the COVID-19 vaccine are needed. -
Citations
Citations to this article as recorded by- Nigerians’ attitudes and perceptions towards vaccine acceptance during and after the COVID-19 pandemic
Jonas Lotanna Ibekwe, Victor Oluwafemi Femi-Lawal, Jolly Akor Thomas, Faith Uzoamaka Okei, Moses Ojomakpenen Ojile, Oluwatobiloba Oladipupo Akingbulugbe
Journal of Medicine, Surgery, and Public Health.2024; 2: 100066. CrossRef - Knowledge, attitudes, and factors determining the willingness for COVID-19 vaccination among students in Bangladesh: An online-based cross-sectional study
Ashis Talukder, Soheli Sharmin, Chuton Deb Nath, Iqramul Haq, Md. Ismail Hossain, Md. Jakaria Habib, Sabiha Shirin Sara
Journal of Public Health.2024; 32(4): 663. CrossRef - Healthcare professionals’ perception and COVID-19 vaccination attitudes in North-Western Ghana: A multi-center analysis
Augustine Ngmenemandel Balegha, Suburu Abdul-Aziz, Louis Mornah, Pracheth Raghuveer
PLOS ONE.2024; 19(2): e0298810. CrossRef - Behavioral Insights from Vaccine Adoption in Nigeria: Cross-Sectional Survey Findings
Sohail Agha, Ifeanyi Nsofor, Drew Bernard, Sarah Francis, Nandan Rao
Interactive Journal of Medical Research.2024; 13: e47817. CrossRef - COVID-19 vaccine hesitancy in Latin America and Africa: a scoping review
Bruna Aparecida Gonçalves, Camila Carvalho de Souza Amorim Matos, Jonathan Vicente dos Santos Ferreira, Renata Fortes Itagyba, Vinicius Rocha Moço, Marcia Thereza Couto
Cadernos de Saúde Pública.2023;[Epub] CrossRef - Suspecting the Figures: What Church Leaders Think About Government’s Commitment to Combating COVID-19 in Nigeria
Uchechukwu M. Agbo, George C. Nche
Journal of Asian and African Studies.2023; 58(5): 725. CrossRef - Access to COVID-19 vaccines and testing in Africa: the importance of COVAX - Nigeria as a case study
Rafaella Fortini Queiroz Grenfell, Oyetunde Timothy Oyeyemi
Pathogens and Global Health.2023; 117(2): 152. CrossRef - COVID-19 vaccine acceptance prediction: The roles of students’ attitude towards science and mathematics and knowledge of COVID-19 pandemic
Sunday Ogbu, Ogochukwu Ebere Emenike, Amaka Loretta Nwankwo
Electronic Journal of Medical and Educational Tech.2023; 16(2): em2304. CrossRef - Factors associated with COVID-19 vaccine hesitancy among healthcare workers in Cameroon and Nigeria: a web-based cross-sectional study
Jerry Brown Aseneh, Valirie Ndip Agbor, Benjamin Momo Kadia, Elvis Anyaehiechukwu Okolie, Chinelo Janefrances Ofomata, Christie Linonge Etombi, Domin Sone M Ekaney, Yvonne Walburga Joko Fru
International Health.2023; 15(6): 702. CrossRef - Willingness to COVID-19 vaccination: Empirical evidence from EU
Imran Ur Rahman, Arslan Austin, Naveed Nelson
Heliyon.2023; 9(5): e15776. CrossRef - Radiographers’ knowledge, attitude and adherence to standard COVID-19 precautions and the policy implications: a national cross-sectional study in Nigeria
Charles Ikechukwu Ezema, Okechukwu Felix Erondu, Ogochukwu Kelechi Onyeso, Chiedozie James Alumona, Andrew Wueseter Ijever, Charity Ndidiamaka Amarachukwu, Amaeze Augustine Amaeze
Annals of Medicine.2023;[Epub] CrossRef - Declining trends in vaccine confidence across sub-Saharan Africa: A large-scale cross-sectional modeling study
A. de Figueiredo, E. Temfack, R. Tajudeen, H. J. Larson
Human Vaccines & Immunotherapeutics.2023;[Epub] CrossRef - Knowledge and acceptance of COVID-19 vaccine among healthcare workers in Enugu metropolis, Enugu state, Nigeria
Kelechi U. Imediegwu, Jude C. Abor, Chiamaka Q. Onyebuchukwu, Hilary I. Ugwu, Ogechi I. Ugwu, Udo Ego Anyaehie, Oluchi A. Onyia
Frontiers in Public Health.2023;[Epub] CrossRef - COVID-19 vaccination acceptance (uptake, hesitancy, intention to receive and timeliness of the intention to receive) and the determinants among health workers in Ebonyi state, Nigeria: an analytical cross-sectional study
Ugwu I Omale, Onyinyechukwu U Oka, Chidinma I Amuzie, Victor U Uduma, Azuka S Adeke, Cordis O Ikegwuonu, Glory E Nkwo, Ugochi I A Nwali, Osarhiemen Iyare, Richard L Ewah, Olaedo O Nnachi, Okechukwu O Ukpabi, Ifeyinwa M Okeke
BMJ Open.2023; 13(7): e068668. CrossRef - Hesitação vacinal contra a COVID-19 na América Latina e África: uma revisão de escopo
Bruna Aparecida Gonçalves, Camila Carvalho de Souza Amorim Matos, Jonathan Vicente dos Santos Ferreira, Renata Fortes Itagyba, Vinicius Rocha Moço, Marcia Thereza Couto
Cadernos de Saúde Pública.2023;[Epub] CrossRef - The Social Ecological Model: A Framework for Understanding COVID-19 Vaccine Uptake among Healthcare Workers—A Scoping Review
Damian Naidoo, Anna Meyer-Weitz, Kaymarlin Govender
Vaccines.2023; 11(9): 1491. CrossRef - Health service factors affecting the COVID-19 vaccination campaign in a Ghanaian metropolis: A qualitative exploratory study
Susanna Aba Aba Abraham, John Oti Amoah, Dorcas Frempomaa Agyare, Deogratias Kaheeru Sekimpi, Diana Bosomtwe-Duker, Andrews Adjei Druye, Gifty Osei Berchie, Dorcas Obiri-Yeboah
BMJ Open.2023; 13(12): e076184. CrossRef - ‘Why Should I Take the COVID-19 Vaccine after Recovering from the Disease?’ A Mixed-methods Study of Correlates of COVID-19 Vaccine Acceptability among Health Workers in Northern Nigeria
Zubairu Iliyasu, Muhammad R. Garba, Auwalu U. Gajida, Taiwo G. Amole, Amina A. Umar, Hadiza M. Abdullahi, Aminatu A. Kwaku, Hamisu M. Salihu, Muktar H. Aliyu
Pathogens and Global Health.2022; 116(4): 254. CrossRef - A Global Map of COVID-19 Vaccine Acceptance Rates per Country: An Updated Concise Narrative Review
Malik Sallam, Mariam Al-Sanafi, Mohammed Sallam
Journal of Multidisciplinary Healthcare.2022; Volume 15: 21. CrossRef - Knowledge, Attitudes, and Perception towards COVID-19 Vaccination among the Adult Population: A Cross-Sectional Study in Turkey
Meliha Cagla Sonmezer, Taha Koray Sahin, Enes Erul, Furkan Sacit Ceylan, Muhammed Yusuf Hamurcu, Nihal Morova, Ipek Rudvan Al, Serhat Unal
Vaccines.2022; 10(2): 278. CrossRef - Factors influencing COVID-19 vaccine uptake among adults in Nigeria
Halimat Adedeji-Adenola, Olubusola A. Olugbake, Shakirat A. Adeosun, Ismaeel Yunusa
PLOS ONE.2022; 17(2): e0264371. CrossRef - Perception and Prevention Practices Relating to Covid 19 Infection Among Elderly in Ogun State, Nigeria
Adenitire G., Agbede C.O.
International Journal of Public Health and Pharmac.2022; 2(1): 29. CrossRef - Predicting nursing students' intention to attend face‐to‐face classes on school reopening: A theory of planned behavior application
Ryan Michael F. Oducado, Jerome V. Cleofas, Gil P. Soriano
Nursing Forum.2022; 57(5): 733. CrossRef - COVID-19 vaccination in Nigeria: A rapid review of vaccine acceptance rate and the associated factors
Oluwatosin Olu-Abiodun, Olumide Abiodun, Ngozi Okafor, Nusirat Elelu
PLOS ONE.2022; 17(5): e0267691. CrossRef - COVID-19 vaccine acceptance among health care workers in Africa: A systematic review and meta-analysis
Martin Ackah, Louise Ameyaw, Mohammed Gazali Salifu, Delali Pearl Afi Asubonteng, Cynthia Osei Yeboah, Eugene Narkotey Annor, Eunice Abena Kwartemaa Ankapong, Hosea Boakye, Muhammad Shahzad Aslam
PLOS ONE.2022; 17(5): e0268711. CrossRef - A national survey of COVID-19 vaccine acceptance in Nigeria
Ahmad Ibrahim Al-Mustapha, Ochulor Okechukwu, Ademola Olayinka, Oyeniyi Rasheed Muhammed, Muftau Oyewo, Samuel A. Owoicho, Ahmed Tijani Abubakar, Abdulsalam Olabisi, Aliyu Jibril, Simon Ereh, Oluwatosin Enoch Fakayode, Oluwaseun Adeolu Ogundijo, Nusirat E
Vaccine.2022; 40(33): 4726. CrossRef - COVID-19 vaccine hesitancy in Africa: a scoping review
Betty B. B. Ackah, Michael Woo, Lisa Stallwood, Zahra A. Fazal, Arnold Okpani, Ugochinyere Vivian Ukah, Prince A. Adu
Global Health Research and Policy.2022;[Epub] CrossRef - COVID-19 Vaccine Acceptance and Associated Factors Among College Students in Dessie City, Northeastern Ethiopia
Gete Berihun, Zebader Walle, Daniel Teshome, Leykun Berhanu, Mohammed Derso
Journal of Multidisciplinary Healthcare.2022; Volume 15: 1735. CrossRef - Career Aspiration Fulfillment and COVID-19 Vaccination Intention among Nigerian Youth: An Instrumental Variable Approach
Abayomi Samuel Oyekale
International Journal of Environmental Research an.2022; 19(16): 9813. CrossRef - COVID-19 Vaccine Attitude and Its Predictors Among People Living With Chronic Health Conditions in Ibadan, Nigeria
Lucia Yetunde Ojewale, Rotimi Felix Afolabi, Adesola Ogunniyi
International Journal of Public Health.2022;[Epub] CrossRef - Associations between COVID-19 vaccine hesitancy and the experience of violence among women and girls living with and at risk of HIV in Nigeria
Morenike Oluwatoyin Folayan, Olujide Arije, Amaka Enemo, Aaron Sunday, Amira Muhammad, Hasiya Yunusa Nyako, Rilwan Mohammed Abdullah, Henry Okiwu, Erik Lamontagne
African Journal of AIDS Research.2022; 21(4): 306. CrossRef - Willingness to receive COVID-19 vaccine: A survey among medical radiation workers in Nigeria
Grace Ben Inah, Samuel Archibong Efanga, Ekaete Vincent Ukpong, Christiana Ifeyinwa Obiora
Calabar Journal of Health Sciences.2022; 6: 80. CrossRef - Acceptance of COVID-19 vaccine among healthcare workers in Africa, systematic review and meta-analysis
Zerihun Figa, Tesfaye Temesgen, Addisu Getnet Zemeskel, Moges Ganta, Asrat Alemu, Mesfin Abebe, Zemachu Ashuro
Public Health in Practice.2022; 4: 100343. CrossRef - Perception and willingness to accept COVID-19 Vaccines: A cross-sectional survey of the general population of Sokoto State, Nigeria
Oche Mansur Oche, Habibullah Adamu, Musa Yahaya, Hudu Garba Illo, Abdulaziz Mohammad Danmadami, Adamu Ijapa, Asmau Mohammad Wali, Hamza Yusuf, Hafsat Muhammad, Abba Aji, Harapan Harapan
PLOS ONE.2022; 17(12): e0278332. CrossRef - COVID-19 vaccination acceptance among community members and health workers in Ebonyi state, Nigeria: study protocol for a concurrent-independent mixed method analyses of intention to receive, timeliness of the intention to receive, uptake and hesitancy to
Ugwu I Omale, Osarhiemen Iyare, Richard L Ewah, Chidinma I Amuzie, Onyinyechukwu U Oka, Victor U Uduma, Azuka S Adeke, Cordis O Ikegwuonu, Olaedo O Nnachi, Okechukwu O Ukpabi, Ifeyinwa M Okeke, Glory E Nkwo, Ugochi IA Nwali
BMJ Open.2022; 12(12): e061732. CrossRef - Drivers of COVID-19 Vaccine Uptake amongst Healthcare Workers (HCWs) in Nigeria
Sohail Agha, Adaobi Chine, Mathias Lalika, Samikshya Pandey, Aparna Seth, Alison Wiyeh, Alyssa Seng, Nandan Rao, Akhtar Badshah
Vaccines.2021; 9(10): 1162. CrossRef
- Nigerians’ attitudes and perceptions towards vaccine acceptance during and after the COVID-19 pandemic
- Behavioral therapy and pharmacotherapy for relapse prevention in abstinent smokers: a rapid review and meta-analysis for the Korea Preventive Service Task Force
- Naae Lee, Eon Sook Lee, Jae Moon Yun, Cheol Min Lee, Seung-Won Oh, Younglee Choi, Belong Cho
- Osong Public Health Res Perspect. 2021;12(4):244-253. Published online July 6, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0017
- 5,945 View
- 94 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Objectives
This study aimed to assess the effectiveness of relapse prevention interventions involving behavioral and pharmacological treatment among abstinent smokers.
Methods
This rapid review was conducted using MEDLINE, Cochrane CENTRAL, CINAHL, Embase, KMbase, and KoreaMed to identify studies published until June 20, 2020. The participants were abstinent smokers who quit smoking on their own, due to pregnancy, hospitalization, or by participating in a smoking cessation program. We found a systematic review that fit the objective of this study and included 81 randomized controlled trials (RCTs). Studies that did not present information on smoking cessation status, had no control group, or used reward-based interventions were excluded. Random effect and fixed effect meta-analyses were used to estimate the relative risk (RR) and 95% confidence interval (CI). In subgroup analyses, differences between subgroups were verified based on the participant setting, characteristics, intervention type, and intensity.
Results
Following screening, 44 RCTs were included in the meta-analysis. The review reported no differences in the success rate of relapse prevention between the behavioral interventions. Pharmacotherapy interventions showed higher success rates (RR, 1.15; 95% CI, 1.05−1.26; I2=40.71%), depending on prior abstinence duration and the drug type. Conclusions: The results indicated that pharmacotherapy has a significant effect on preventing relapse among abstinent smokers. -
Citations
Citations to this article as recorded by- A Survey of the Clinical Practice of Korean Medicine for Smoking Cessation in Public Health Centers: A Web-Based Survey of Public Health Doctors of Korean Medicine
Gyoungeun Park, Jeong-Hyun Moon, Eun-Jung Kim, Byung-Kwan Seo, Yong-Hyeon Baek, Won-Suk Sung
Perspectives on Integrative Medicine.2024; 3(1): 45. CrossRef
- A Survey of the Clinical Practice of Korean Medicine for Smoking Cessation in Public Health Centers: A Web-Based Survey of Public Health Doctors of Korean Medicine
- Validity and reliability of the Health-Related Quality of Life Instrument with 8 Items (HINT-8) in Korean breast cancer patients
- Juyoung Kim, Min-Woo Jo, Hyeon-Jeong Lee, Sei-Hyun Ahn, Byung Ho Son, Jong Won Lee, Sae Byul Lee
- Osong Public Health Res Perspect. 2021;12(4):254-263. Published online August 5, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0005
- 6,574 View
- 126 Download
- 6 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Objectives
This study evaluated the validity and reliability of the Health-Related Quality of Life Instrument with 8 Items (HINT-8) in postoperative breast cancer patients in South Korea.
Methods
The study included 300 breast cancer patients visiting a tertiary hospital. We measured health-related quality of life (HRQoL) using the HINT-8, the 5-level EQ-5D version (EQ-5D-5L), and the Functional Assessment of Cancer Therapy-Breast (FACT-B). Discriminatory ability, known-group validity, and convergent validity were assessed. Reliability was evaluated with the Cohen kappa, weighted kappa, and intraclass correlation coefficient (ICC).
Results
The EQ-5D-5L indexes (p<0.001) and EQ visual analogue scale (VAS) scores (p<0.001) were significantly higher in subjects with no problems in each item of the HINT-8 than in those with problems. The FACT-B total scores were also higher in subjects without problems on the HINT-8. Older age, lower education level, and comorbidities were associated with a lower HINT-8 index. The HINT-8 index was correlated with the EQ-5D-5L index and the EQ VAS, with correlation coefficients of 0.671 (p<0.001) and 0.577 (p<0.001), respectively. The correlation coefficients between the HINT-8 and the FACT-B ranged from 0.390 to 0.714. The ICC was 0.690 (95% confidence interval, 0.580–0.780).
Conclusion
The HINT-8 showed appropriate validity for capturing HRQoL in postoperative breast cancer patients. -
Citations
Citations to this article as recorded by- Health-related quality of life of premenopausal young breast cancer survivors undergoing endocrine therapy
Kyungmi Lee, Hye Suk Jun
European Journal of Oncology Nursing.2024; 68: 102496. CrossRef - Smartphone application-based rehabilitation in patients with chronic respiratory and cardiovascular diseases
Chiwook Chung, Ah-Ram Kim, Dongbum Kim, Hee Kwon, Seong Ho Lee, Il-Young Jang, Min-Woo Jo, Do-Yoon Kang, Sei Won Lee
Scientific Reports.2024;[Epub] CrossRef - Willingness to pay for integrative healthcare services to treat sleep disturbances: Evidence from a nationwide survey
Min Kyung Hyun
European Journal of Integrative Medicine.2023; 58: 102223. CrossRef - Internal Structure of the Health-Related Quality of Life Instrument with 8-Items in a Nationally Representative Population
Eun-Hyun Lee
Journal of Korean Academy of Nursing.2023; 53(3): 359. CrossRef - Factors influencing health-related quality of life for young single-person households: the mediating effect of resilience
Soo Jin Lee, Sujin Lee, Xianglan Jin
Journal of Korean Biological Nursing Science.2023; 25(3): 160. CrossRef - Smartphone application-based rehabilitation in patients with chronic respiratory and cardiovascular diseases: a randomised controlled trial study protocol
Chiwook Chung, Ah-Ram Kim, Il-Young Jang, Min-Woo Jo, Seongho Lee, Dongbum Kim, Hee Kwon, Do-Yoon Kang, Sei Won Lee
BMJ Open.2023; 13(9): e072698. CrossRef - Health-related quality of life among cancer patients and survivors and its relationship with current employment status
Woorim Kim, Kyu-Tae Han, Seungju Kim
Supportive Care in Cancer.2022; 30(5): 4547. CrossRef - Associations between Food Groups and Health-Related Quality of Life in Korean Adults
Shamirah Nabbosa, Sunghee Lee
Nutrients.2022; 14(17): 3643. CrossRef - Validity of the Health-Related Quality of Life Instrument with 8 Items (HINT-8) in the Korean Elderly: A Cross-Sectional Study
Seon-Ha Kim, Miok Kim
Journal of Korean Gerontological Nursing.2022; 24(3): 248. CrossRef
- Health-related quality of life of premenopausal young breast cancer survivors undergoing endocrine therapy
Brief Reports
- COVID-19 vaccine safety monitoring in the Republic of Korea: February 26, 2021 to April 30, 2021
- Hyun-kyung Oh, Eun Kyeong Kim, Insob Hwang, Tae Eun Kim, Yeon-kyeong Lee, Eunju Lee, Yeon-Kyeng Lee
- Osong Public Health Res Perspect. 2021;12(4):264-268. Published online August 13, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0157
- 5,928 View
- 142 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Objectives
On February 26, 2021, coronavirus disease 2019 (COVID-19) vaccination was started for high-priority groups based on the recommendation of the Advisory Committee on Immunization Practices with 2 available COVID-19 vaccines (AstraZeneca and Pfizer-BioNTech) in Korea. This report provides a summary of adverse events following COVID-19 vaccination as of April 30, 2021.
Methods
Adverse events following immunization are notifiable by medical doctors to the Korea Immunization Management System (KIMS) under the national surveillance system. We analyzed all adverse events reports following COVID-19 vaccination to the KIMS from February 26 to April 30, 2021.
Results
In total, 16,196 adverse events following 3,586,814 administered doses of COVID-19 vaccines were reported in approximately 2 months (February 26 to April 30, 2021). Of these, 15,658 (96.7%) were non-serious adverse events, and 538 (3.3%) were serious adverse events, including 73 (0.5%) deaths. The majority of adverse events (n=13,063, 80.7%) were observed in women, and the most frequently reported adverse events were myalgia (52.2%), fever (44.9%), and headache (34.9%). Of the 73 deaths following the COVID-19 vaccination, none were related to the vaccines.
Conclusion
By April 30, 3.6 million doses of the COVID 19 vaccine had been given in Korea, and the overwhelming majority of reports were for non-serious events. The Korea Disease Control and Prevention Agency continues to monitor the safety of COVID-19 vaccination. -
Citations
Citations to this article as recorded by- A prospective cohort study protocol: monitoring and surveillance of adverse events following heterologous booster doses of Oxford AstraZeneca COVID-19 vaccine in previous recipients of two doses of Sinopharm or Sputnik V vaccines in Iran
Shahin Soltani, Behzad Karami Matin, Mohammad Mehdi Gouya, Sayed Mohsen Zahraei, Ghobad Moradi, Omid Chehri, Moslem Soofi, Mehdi Moradinazar, Fatemeh Khosravi Shadmani, Mahsa Kalantari, Hamidreza Khajeha, Mohammad Hassan Emamian, Farid Najafi
BMC Public Health.2023;[Epub] CrossRef - Herpes Zoster Reactivation After mRNA and Adenovirus-Vectored Coronavirus Disease 2019 Vaccination: Analysis of National Health Insurance Database
Jin Gu Yoon, Young-Eun Kim, Min Joo Choi, Won Suk Choi, Yu Bin Seo, Jaehun Jung, Hak-Jun Hyun, Hye Seong, Eliel Nham, Ji Yun Noh, Joon Young Song, Woo Joo Kim, Dong Wook Kim, Hee Jin Cheong
The Journal of Infectious Diseases.2023; 228(10): 1326. CrossRef - Safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents
Young June Choe, Seonju Yi, Insob Hwang, Jia Kim, Young-Joon Park, Eunhee Cho, Myoungyoun Jo, Hyunju Lee, Eun Hwa Choi
Vaccine.2022; 40(5): 691. CrossRef - Direct and Indirect Associations of Media Use With COVID-19 Vaccine Hesitancy in South Korea: Cross-sectional Web-Based Survey
Minjung Lee, Myoungsoon You
Journal of Medical Internet Research.2022; 24(1): e32329. CrossRef - Self-Reported COVID-19 Vaccines’ Side Effects among Patients Treated with Biological Therapies in Saudi Arabia: A Multicenter Cross-Sectional Study
Lama T AlMutairi, Wesal Y Alalayet, Sondus I Ata, Khalidah A Alenzi, Yazed AlRuthia
Vaccines.2022; 10(6): 977. CrossRef - COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021
Insob Hwang, Kyeongeun Park, Tae Eun Kim, Yunhyung Kwon, Yeon-Kyeng Lee
Osong Public Health and Research Perspectives.2021; 12(6): 396. CrossRef
- A prospective cohort study protocol: monitoring and surveillance of adverse events following heterologous booster doses of Oxford AstraZeneca COVID-19 vaccine in previous recipients of two doses of Sinopharm or Sputnik V vaccines in Iran
- The laboratory test procedure to confirm rotavirus vaccine infection in severe complex immunodeficiency patients
- Su-Jin Chae, Seung-Rye Cho, Wooyoung Choi, Myung-Guk Han, Deog-Yong Lee
- Osong Public Health Res Perspect. 2021;12(4):269-273. Published online August 13, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0079
- 4,603 View
- 87 Download
-
 Abstract
Abstract
 PDF
PDF - The rotavirus vaccine is a live vaccine, and there is a possibility of infection by the virus strain used in the vaccine. We investigated the process of determining whether an infection was caused by the vaccine strain in a severe complex immunodeficiency (SCID) patient with rotavirus infection. The patient was vaccinated with RotaTeq prior to being diagnosed with SCID. The testing process was conducted in the following order: confirming rotavirus infection, determining its genotype, and confirming the vaccine strain. Rotavirus infection was confirmed through enzyme immunoassay and VP6 gene detection. G1 and P[8] were identified by multiplex polymerase chain reaction for the genotype, and G3 was further identified using a single primer. By detecting the fingerprint gene (WC3) of RotaTeq, it was confirmed that the detected virus was the vaccine strain. Genotypes G1 and P[8] were identified, and the infection was suspected of having been caused by rotavirus G1P[8]. G1P[8] is the most commonly detected genotype worldwide and is not included in the recombinant strains used in vaccines. Therefore, the infection was confirmed to have been caused by the vaccine strain by analyzing the genetic relationship between VP4 and VP7. Rotavirus infection by the vaccine strain can be identified through genotyping and fingerprint gene detection. However, genetic linkage analysis will also help to identify vaccine strains.





 First
First Prev
Prev


