Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 13(6); 2022 > Article
-
Original Article
mRNA vaccine effectiveness against SARS-CoV-2 B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant transmission from home care cases to household contacts in South Korea -
Hanul Park1
 , Young Joon Park1
, Young Joon Park1 , Sang Eun Lee1
, Sang Eun Lee1 , Min Jei Lee1
, Min Jei Lee1 , Hyungtae Ahn2
, Hyungtae Ahn2
-
Osong Public Health and Research Perspectives 2022;13(6):435-442.
DOI: https://doi.org/10.24171/j.phrp.2022.0243
Published online: November 28, 2022
1Division of Epidemiological Investigation Analysis, Korea Disease Control and Prevention Agency, Cheongju, Korea
2Division of Emerging Infectious Disease Response, Korea Disease Control and Prevention Agency, Cheongju, Korea
- Corresponding author: Young Joon Park Division of Epidemiological Investigation Analysis, Korea Disease Control and Prevention Agency, 187 Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju 28159, Korea E-mail: pahmun@korea.kr
© 2022 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- Household contacts of confirmed cases of coronavirus disease 2019 (COVID-19) are exposed to a high risk of viral transmission, and secondary incidence is an important indicator of community transmission. This study analyzed the secondary attack rate and mRNA vaccine effectiveness against transmission (VET) for index cases (patients treated at home) confirmed to be infected with the Delta and Omicron variants.
-
Methods
- The subjects of the study were 4,450 index cases and 10,382 household contacts. Logistic regression analysis was performed to compare the secondary attack rate by vaccination status, and adjusted relative risk and 95% confidence intervals were identified.
-
Results
- The secondary attack rate of the Delta variant was 27.3%, while the secondary attack rate of the Omicron variant was 29.8%. For the Delta variant, groups with less than 90 days and more than 90 days after 2 doses of mRNA vaccination both showed a VET of 37%. For the Omicron variant, a 64% VET was found among those with less than 90 days after 2 doses of mRNA vaccination.
-
Conclusion
- This study provides useful data on the secondary attack rate and VET of mRNA vaccines for household contacts of COVID-19 cases in South Korea.
- A new variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported to the World Health Organization (WHO) from South Africa in November 2021. This variant is characterized by its potential immune escape and high transmission rate. The WHO designated this a variant of concern, naming it Omicron [1,2]. As of April 1, 2022, the Omicron variant was further classified as follows: BA.1.1.529, BA.1, BA.2, and BA.3 [3]. As of April 5, 2022, the Health and Safety Executive (UK) reported 1,125 cases of XE, a mix of the BA.1 and BA.2 sub-variants [4].
- In Korea, the first case of the Omicron variant was detected on November 30, 2021 [5]. On January 24, 2022, the predominant variant in Korea changed from the Delta variant to the Omicron variant [6]. As of January 3, 2022, 83.0% of Korea’s population had received vaccines against coronavirus disease 2019 (COVID-19) [7].
- A South African study reported that confirmed cases of the Omicron variant increased by approximately 2-fold compared with the previous number of confirmed cases and that the basic reproduction number of the Omicron variant could increase up to 4.2-fold compared with that of the Delta variant [8]. A study conducted at 9 nursing homes in Korea reported that the incidence rate of the Omicron variant was 11.18-fold higher than that of the Delta variant [9]. Previous studies have indicated that a reduction in vaccine effectiveness (VE) due to immune escape may have contributed to the higher incidence rates of the Omicron variant [10,11]. Furthermore, previous studies have reported that the vaccine effectiveness against transmission (VET) was lower for the Omicron variant than for the Delta variant [12,13].
- Studies from the UK and Denmark have reported that identifying VE and quantifying VET are essential for estimating the transmission of new variants [14,15]. A recent study conducted among 10 million inhabitants in Korea showed the effectiveness of 3 doses of the mRNA vaccine against infection [16]. The household contacts of confirmed cases of COVID-19 are at a high risk of viral transmission [14]. In the UK, the incidence rates of infected household members increased concomitantly with the increased incidence rate of confirmed cases of the Delta variant [17]. Thus, the secondary attack rate (SAR) among household contacts is an important indicator of community transmission [18]. This study aimed to identify the SAR and mRNA VET among index cases (patients treated at home) with laboratory-confirmed cases of the Delta and Omicron variants.
Introduction
- Participants
- This study included 4,450 index COVID-19 cases with either the Delta or Omicron variant, as confirmed by whole genome sequencing (WGS) in the laboratory, and their 10,382 household contacts from June 25, 2021, to January 22, 2022. Index cases were defined as those who had positive results on a SARS-CoV-2 polymerase chain reaction (PCR) test and were being isolated and treated at home. Confirmed cases were monitored for their health twice a day via phone calls or a mobile phone application, and if necessary, they had a telephone consultation and obtained a prescription for medication. The household contacts of the index cases were defined as those who were registered in the contact management system of the Korea Disease Control and Prevention Agency (KDCA).
- When the Delta variant became predominant, household contacts were isolated at home for 14 days from the last day of contact with index cases, and they were required to undergo PCR tests at a public health center when they were made aware that they were contacts, if they became symptomatic during isolation, and when they were released from isolation [19].
- When the Omicron variant became predominant, household contacts were isolated at home for 10 days, unless they met all of the following criteria at the time of contact with index cases: (1) fully vaccinated at the time of close contact; (2) asymptomatic; and (3) not residents, users, or employees at high-risk facilities, such as long-term care facilities. Household contacts had PCR tests at a public health center near their jurisdiction on the day when they became aware that they were contacts of COVID-19 cases and 6 to 7 days after their last contact with confirmed index cases [20]. Moreover, the staff of the public health center educated all household contacts about home-quarantine guidelines.
- Data were obtained from a total of 44,573 index cases and 97,300 household contacts for analysis. Those who met the following criteria were excluded: did not undergo a test for a variant, had other variants, had incorrectly registered information, had unidentifiable vaccination status (VS); underwent a diagnostic test ≥15 days after the date when the index case was diagnosed, and had non-mRNA vaccines (Figure 1). VS was defined as at least 14 days having passed after a certain number of vaccinations. All index cases had received mRNA vaccines (Pfizer or Moderna) and the duration post-vaccination was analyzed using a cut-off of 90 days after vaccination.
- Data Sources
- Data on home healthcare and WGS of index cases were obtained from the COVID-19 patient management information system of the KDCA. Information about household contacts was obtained from the COVID-19 information management system of the KDCA. Data on VS were obtained from the COVID-19 vaccination system of the KDCA.
- Statistical Analysis
- Descriptive statistics were used to analyze participants’ demographic characteristics and SAR, which were expressed as percentages (%). Logistic regression analysis was performed to compare the SAR according to the number of vaccine doses, and the adjusted related risk (aRR) was estimated. In an analysis model, sex, age, VS, and the diagnosis date of index cases and household contacts were adjusted. A p-value <0.05 was considered to indicate statistical significance, and a 95% confidence interval (CI) was presented. All data were analyzed using the R software ver. 4.1.2 (The R Foundation, Vienna, Austria).
- IRB/IACUC Approval
- Information about all study participants was obtained after obtaining consent based on the Infectious Diseases Control and Prevention Act. The present study was reviewed and approved by the Institutional Review Board of the KDCA (2022-05-02-PE-A).
Materials and Methods
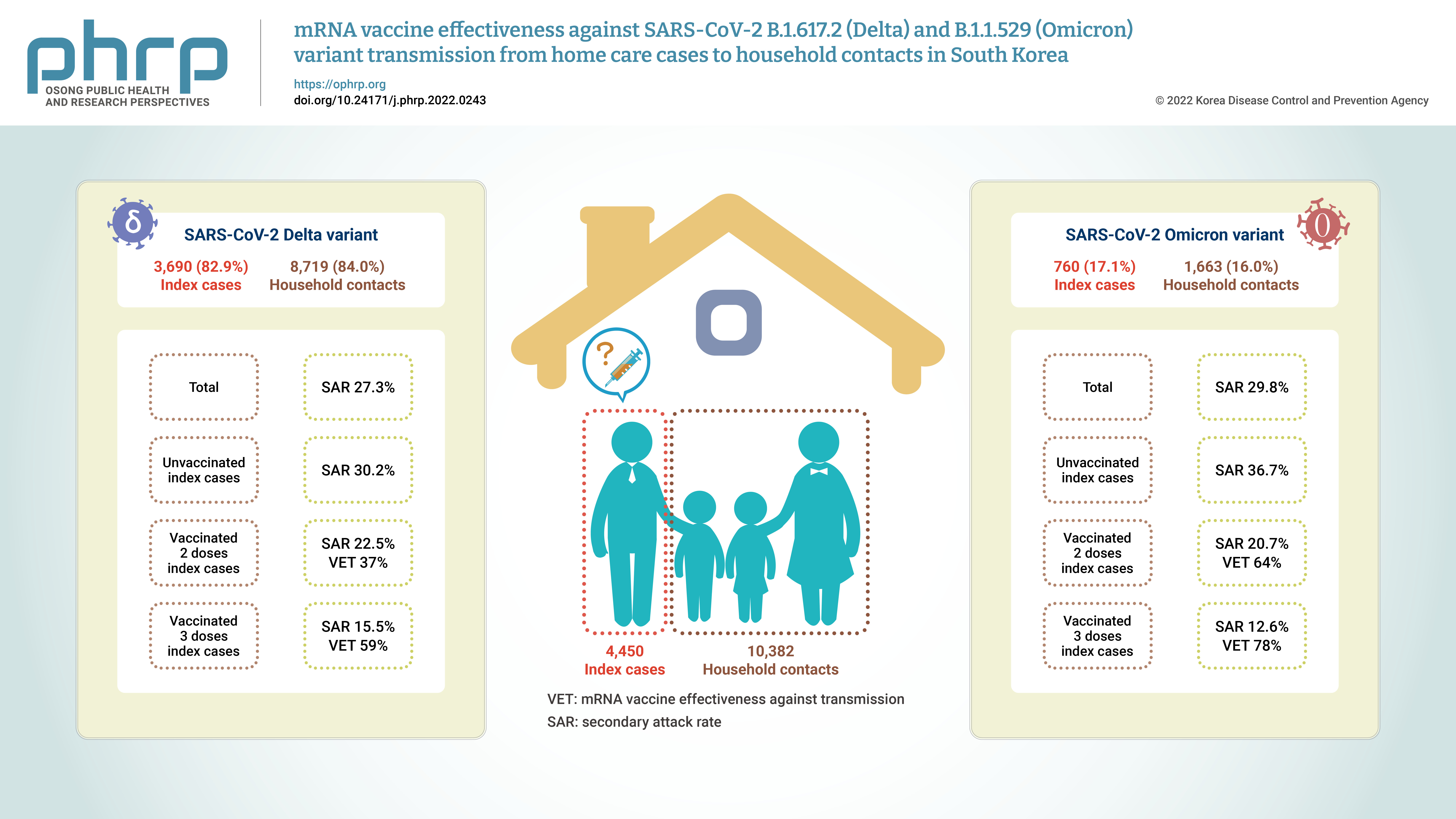
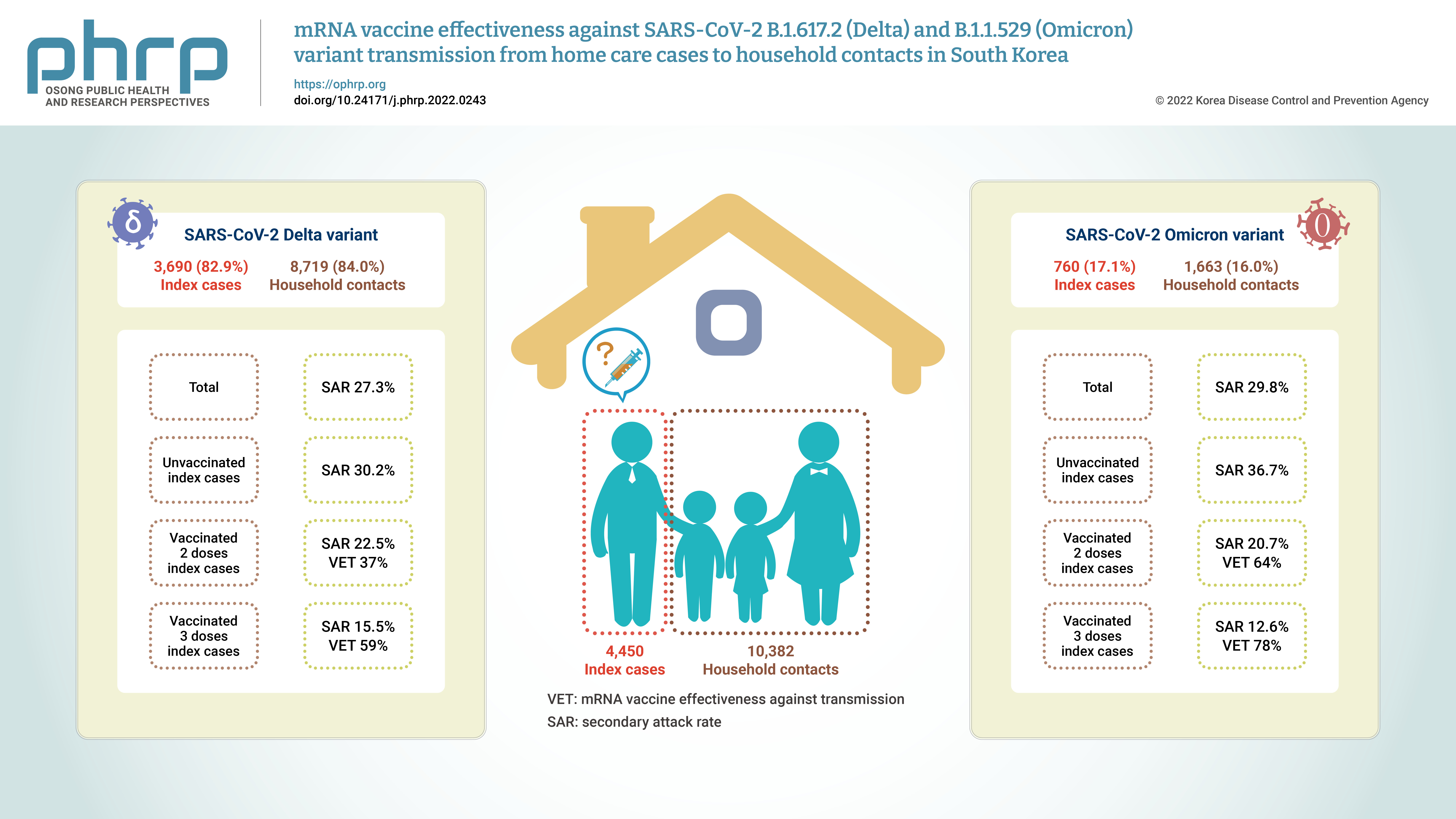
- Of the total of 14,832 participants, 4,450 (30.0%) were index cases and 10,382 (70.0%) were household contacts. Among both index cases and household contacts, the proportion of women was higher than that of men. The largest age group among index cases was ≤19 years (40.7%), whereas 37.1% of household contacts were in the age group of 30 to 49 years. In terms of VS, unvaccinated status predominated among the index cases, whereas the most common status among the household contacts was 2 doses of an mRNA vaccine (≤90 days) (Tables 1, 2).
- Among household contacts, 2,877 (27.7%) participants were confirmed cases. The SAR of female index cases was 27.8%, which was higher than that of male cases. The index cases aged ≥75 years showed the highest SAR (38.3%). In an analysis of the index group according to VS, the unvaccinated group showed the highest SAR (30.8%), and the group that received the third dose of the vaccine showed the lowest SAR (13.6%). The aRR was estimated to identify risk factors for secondary transmission and the VET for index cases. In terms of age, the aRR was 1.87 (95% CI, 1.23–2.84) for individuals aged ≥75 years and 0.62 (95% CI, 0.54–0.70) for those aged ≤19 years compared to the age group of 30–49 years. In the association of VS with secondary transmission, the aRR of 1 dose was 0.68 (95% CI, 0.54–0.87), that of 2 doses (≤90 days) was 0.56 (95% CI, 0.48–0.65), that of 2 doses (>90 days) was 0.68 (95% CI, 0.58–0.79), and that of 3 doses was 0.28 (95% CI, 0.18–0.46) compared to the unvaccinated group.
- Among the total of 4,450 index cases, 3,690 (82.9%) were infected with the Delta variant, and they had 8,719 (84.0%) household contacts. Among these, 2,382 confirmed cases were found, corresponding to a SAR of 27.3%. The index cases aged ≥75 years showed the highest SAR (38.2%). In an analysis according to VS, the unvaccinated group showed the highest SAR (30.2%), and the group that received the third dose of the vaccine showed the lowest SAR (15.5%). We estimated the aRR to identify risk factors for secondary transmission and VET among index cases confirmed to have the Delta variant. In terms of age, the aRR was 2.04 (95% CI, 1.32–3.17) for individuals aged ≥75 years and 0.67 (95% CI, 0.58–0.78) for those aged ≤19 years compared to the age group of 30 to 49 years. The aRRs of 1 dose, 2 doses (≤90 days), 2 doses (>90 days), and 3 doses were 0.70 (95% CI, 0.54–0.89), 0.63 (95% CI, 0.53–0.74), 0.63 (95% CI, 0.52–0.77), and 0.41 (95% CI, 0.19–0.87), respectively, compared with the unvaccinated group.
- Among the total of 4,450 index cases, 760 (17.1%) were infected with the Omicron variant. They had 1,663 (16%) household contacts, of whom 495 (29.8%) participants were confirmed to have become infected. The SAR for female index cases was 30.1%, which was higher than that of male index cases. The index cases aged ≥75 years showed the highest SAR (40%). Unvaccinated index cases showed the highest SAR (36.7%), and patients who had received the third dose of the vaccine showed the lowest SAR (12.6%). The aRR was estimated to identify risk factors for secondary transmission and VET among index cases confirmed to have the Omicron variant.
- The aRR was 0.44 (95% CI, 0.31–0.64) in the index cases aged 20 to 29 years and 0.49 (95% CI, 0.35–0.67) in those aged ≤19 years compared to the index cases aged 30 to 49 years. In terms of the VS of index cases, the aRR was 0.36 (95% CI, 0.25–0.51) in the group that had received the second dose (≤90 days) and 0.22 (95% CI, 0.11–0.42) in the group that had received the third dose compared to the unvaccinated group. In the Omicron group, the aRRs for those who had received the first dose and the second dose >90 days ago were not statistically significant (Figure 2; Table S1–S3).
Results
- This study confirmed the SAR and VET for index cases infected with the Delta or Omicron COVID-19 variants in terms of transmission to their household contacts. The SAR of index cases infected with the Delta variant was 27.3%, while that of index cases infected with the Omicron variant was 29.8%. Among the index cases infected with the Delta variant, the VET was approximately 37% in cases who had received the second dose and approximately 59% in those who had received the third dose compared to the unvaccinated group. Among index cases infected with the Omicron variant, the VET was approximately 64% in those who had received the second dose (≤90 days) and approximately 78% in those who had received the third dose compared to the unvaccinated group. Our results can be used as evidence on the SAR and VET for household contacts of index cases infected with the Delta or Omicron variant in Korea, supporting the evidence from previous international and domestic studies.
- Our study confirmed that the SAR of the Omicron variant was approximately 2.5 percentage points higher than that of the Delta variant. Since the first confirmed case of the Omicron variant, the number of confirmed cases has been rapidly increasing [6]. Our study results are in accordance with the findings of studies conducted in other countries that the emergence of the Omicron variant led to a more rapid increase in the number of confirmed COVID-19 cases than observed with the Delta variant [8,21]. Previous studies have reported that the SAR for the Delta variant was 11% to 36% [15,22,23], while the SAR for the Omicron variant was 51% to 52.7% [23,24]. The SAR for the Delta variant was approximately 1.7-fold higher than that of the Alpha variant [17], and SAR for the Omicron variant was approximately 1.41- to 1.48-fold higher than that of the Delta variant [23,25]. According to a meta-analysis that summarized 54 studies of secondary transmission to household contacts from October 2020 to June 2021, the factors affecting the increased SAR included the development of diagnostic tools, improvements in diagnostic procedures, and an increase in the viral transmission of variants as the COVID-19 pandemic continued [26]. Considering that only a laboratory of the KDCA could identify the Omicron variant until December 30, 2022 in Korea [27], the SAR of the Omicron variant in the community may have been much higher than indicated by our study results.
- In our study, the SAR for index cases aged <19 years with the Delta or Omicron variant was approximately 33% or 51% lower, respectively, than that noted for patients aged 30 to 49 years. This is similar to a previous study's result, wherein the SAR for adults was approximately 2.2-fold higher than that for children [28]. Our study results may be related to the lower rate of vaccination among those <19 years during the time when the Delta variant became predominant in Korea [7]. Similarly, in the present study, the SAR for Delta variant-infected index cases aged ≥75 years was approximately 2.04-fold higher than that for cases aged 30 to 49 years. A previous study reported that the SAR for cases aged 70 to 79 years and ≥80 years was higher than that of the cases aged 30 to 39 years, which was similar to the result of this study [25]. As age increases, individuals tend to spend more time at home. Thus, the household contacts of index cases were more likely to be exposed to the virus for a longer period. Furthermore, these findings could support the hypothesis that exposure to a high viral load for a long period is associated with a high incidence rate [29].
- Our study estimated and compared the SAR among household contacts according to the number of vaccine doses received by the index cases. The SARs of the Delta and Omicron variants were 30.2% and 36.7%, respectively, for unvaccinated index cases. A previous study reported that the SARs of the Delta and Omicron variants among household contacts were 38% and 57%, respectively, for unvaccinated index cases, which was higher than our study results [23]. The SAR of the Omicron variant was 1.51-fold higher than that of the Delta variant. In addition, another study that estimated the SAR of the Omicron variant according to the number of vaccine doses reported higher SARs for unvaccinated index cases than for vaccinated cases (second and third doses), consistent with the results of the present study [24].
- In the present study, statistically significant VET was shown in the mRNA-vaccinated group compared to the unvaccinated group. Index cases with the Delta variant who received 2 doses of mRNA vaccine within ≤90 days or >90 days showed a VET of 37%, while index cases with the Omicron variant who received a dose of mRNA vaccine within ≤90 days showed a VET of 64%. A Norwegian study of the Delta variant reported that the VET in index cases who received the second vaccine dose was 37% regardless of the type of vaccine when compared to the unvaccinated group [23]. A Danish study reported a VET of 42%, which is similar to the results of the present study [15].
- Furthermore, the present study confirmed the VET in index cases who had received the third dose of mRNA vaccine (within ≤90 days) compared to the unvaccinated group. The VET among index cases infected with the Delta and Omicron variants was 59% and 78%, respectively. Previous studies have reported reduced VE against the Omicron variant due to immune escape [10−12]. A study conducted in Colombia reported that the odds ratio for 3 doses of mRNA vaccines versus unvaccinated status was 0.33 for the Omicron variant and 0.065 for the Delta variant, confirming VE [13]. A Korean study reported VE in individuals aged ≥60 years who received 3 doses of mRNA vaccines [16]. VE has been reported in those who received 3 doses of mRNA vaccines, but further studies on VET should be conducted. The present study results support the findings of other studies that a third dose can enhance humoral immunity due to antibody boosting [30,31]. A previous study reported that the risk of infection increased beyond 90 days after receiving an mRNA vaccine [32]. Further studies on VET by the type of the vaccine and the duration post-vaccination should be conducted.
- This study has some limitations. First, since tests to identify the variants were not carried out in all confirmed cases in South Korea, representativeness might be a concern. However, to ensure the representativeness of participants, we carried out tests to identify the variants using randomization. Second, despite frequent household contacts, infection risk-related factors such as the number of rooms in a house, compliance with face mask-wearing, and the degree of physical distancing were not adjusted because no information was available on these variables.
Discussion
Supplementary Material
Table S1.
Table S2.
Table S3.
-
Ethics Approval
This study was approved by the Institutional Review Board of Korea Disease Control and Prevention Agency (2022-05-02-PE-A) and performed in accordance with the principles of the Declaration of Helsinki.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available. If you have any questions about this study, please contact the corresponding author (pahmun@korea.kr).
-
Authors’ Contributions
Conceptualization: YJP, HP; Data curation: HP, MJL, HA; Formal analysis: HP; Methodology: YJP, HP, SEL; Project administration: HP; Visualization: YJP, HP; Writing–original draft: HP; Writing–review & editing: all authors.
Article information
-
Acknowledgements
- The authors appreciate the Laboratory Analysis Team of Korea Disease Control and Prevention Agency (KDCA) for making this study possible.


- 1. World Health Organization (WHO). Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern [Internet]. Geneva: WHO; 2021 [cited 2022 Mar 20]. Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- 2. World Health Organization (WHO). Tracking SARS-CoV-2 variants [Internet]. Geneva: WHO; 2021 [cited 2022 Mar 20]. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants.
- 3. World Health Organization (WHO). Enhancing response to Omicron SARS-CoV-2 variant [Internet]. Geneva: WHO; 2022. [cited 2022 Mar 20]. Available from: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states.
- 4. UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 40 [Internet]. London: UK Health Security Agency; 2022 [cited 2022 Mar 20]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1067672/Technical-Briefing-40-8April2022.pdf.
- 5. Korea Disease Control and Prevention Agency (KDCA). Emergency implementation to block inflow into Korea and prevent transmission of a total of 5 confirmed cases of Omicron mutant virus in Korea [Internet]. Cheongju: KDCA; 2021 [cited 2022 Mar 20]. Available from: http://ncov.mohw.go.kr/upload/viewer/skin/doc.html?fn=1638364982419_20211201222302.pdf&rs=/upload/viewer/result/202205/. Korean.
- 6. Korea Disease Control and Prevention Agency (KDCA). Characteristic analysis of Omicron variations [Internet]. Cheongju: KDCA; 2022 [cited 2022 Mar 20]. Available from: http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6313&contSeq=6313&board_id=312&gubun=BDJ. Korean.
- 7. Korea Disease Control and Prevention Agency (KDCA). COVID-19 immunization dashboard [Internet]. Cheongju: KDCA; 2022 [cited 2022 Jan 3]. Available from: https://ncv.kdca.go.kr. Korean.
- 8. National Institute for Communicable Diseases. The daily COVID-19 effective reproductive number (R) in South Africa [Internet]. Johannesburg: National Institute for Communicable Diseases; 2021 [cited 2022 Mar 20]. Available from: https://www.nicd.ac.za/wp-content/uploads/2021/12/COVID-19-Effective-Reproductive-Number-in-South-Africa-week-48.pdf.
- 9. Park H, Lee JJ, Choi JH, et al. Incidence and fatality rates of SARS-CoV-2 Omicron variant compared with Delta variant in long term care facilities. Public Health Wkly Rep 2022;15:1426−34. Korean.
- 10. Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022;602:654−6.ArticlePubMedPDF
- 11. Lu L, Mok BW, Chen LL, et al. Neutralization of severe acute respiratory syndrome coronavirus 2 Omicron variant by sera from BNT162b2 or CoronaVac vaccine recipients. Clin Infect Dis 2022;75:e822−6.ArticlePubMedPMCPDF
- 12. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) variant: United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1731−4.ArticlePubMedPMC
- 13. Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 2022;327:639−51.ArticlePubMedPMC
- 14. Harris RJ, Hall JA, Zaidi A, et al. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med 2021;385:759−60.ArticlePubMedPMC
- 15. Lyngse FP, Molbak K, Denwood M, et al. Effect of vaccination on household transmission of SARS-CoV-2 Delta variant of concern. Nat Commun 2022;13:3764. ArticlePubMedPMCPDF
- 16. Kim J, Choe YJ, Jang EJ, et al. Effectiveness of booster mRNA vaccines against SARS-CoV-2 infection in an elderly population, South Korea, October 2021-January 2022. Clin Infect Dis 2022;75:920−1.ArticlePubMedPMCPDF
- 17. Allen H, Vusirikala A, Flannagan J, et al. Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): national case-control study. Lancet Reg Health Eur 2022;12:100252. ArticlePubMedPMC
- 18. Haroon S, Chandan JS, Middleton J, et al. COVID-19: breaking the chain of household transmission. BMJ 2020;370:m3181. ArticlePubMed
- 19. Korea Disease Control and Prevention Agency (KDCA). Guidelines for response to coronavirus disease-19 (for local government). ver. 10-1 (2021 Aug 30). Cheongju: KDCA; 2022. Korean.
- 20. Korea Disease Control and Prevention Agency (KDCA). Guidelines for response to coronavirus disease-19 (for local government). ver. 10-3 (2022 Jan 3). Cheongju: KDCA; 2022. Korean.
- 21. UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 35 [Internet]. London: UK Health Security Agency; 2021 [cited 2022 Mar 20]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050999/Technical-Briefing-35-28January2022.pdf.
- 22. de Gier B, Andeweg S, Backer JA, et al. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), the Netherlands, August to September 2021. Euro Surveill 2021;26:2100977. PubMedPMC
- 23. Jalali N, Brustad HK, Frigessi A, et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat Commun 2022;13:5706. ArticlePubMedPMCPDF
- 24. Baker JM, Nakayama JY, O’Hegarty M, et al. SARS-CoV-2 B.1.1.529 (Omicron) variant transmission within households: four U.S. Jurisdictions, November 2021-February 2022. MMWR Morb Mortal Wkly Rep 2022;71:341−6.ArticlePubMedPMC
- 25. Allen H, Tessier E, Turner C, et al. Comparative transmission of SARS-CoV-2 Omicron (B. 1.1. 529) and Delta (B. 1.617. 2) variants and the impact of vaccination: national cohort study, England. medRxiv [Preprint] 2022 Feb 17. https://doi.org/10.1101/2022.02.15.22271001.
- 26. Madewell ZJ, Yang Y, Longini IM Jr, et al. Factors associated with household transmission of SARS-CoV-2: an updated systematic review and meta-analysis. JAMA Netw Open 2021;4:e2122240.ArticlePubMedPMC
- 27. Korea Disease Control and Prevention Agency (KDCA). From December 30th, local governments can identify Omicrons [Internet]. Cheongju: KDCA; 2022 [cited 2022 Mar 20]. Available from: https://ncov.kdca.go.kr/?fn=1640309879513_20211224103759.pdf&rs=/upload/viewer/result/202205/. Korean.
- 28. Bhatt M, Plint AC, Tang K, et al. Household transmission of SARS-CoV-2 from unvaccinated asymptomatic and symptomatic household members with confirmed SARS-CoV-2 infection: an antibody-surveillance study. CMAJ Open 2022;10:E357−66.ArticlePubMedPMC
- 29. Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021;372:eabg3055.ArticlePubMedPMC
- 30. Romero-Olmedo AJ, Schulz AR, Hochstatter S, et al. Dynamics of humoral and T-cell immunity after three BNT162b2 vaccinations in adults older than 80 years. Lancet Infect Dis 2022;22:588−9.ArticlePubMedPMC
- 31. Guerrera G, Picozza M, D’Orso S, et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci Immunol 2021;6:eabl5344.ArticlePubMed
- 32. Israel A, Merzon E, Schaffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ 2021;375:e067873.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Household secondary attack rates and risk factors during periods of SARS-CoV-2 Delta and Omicron variant predominance in the Republic of Korea
Jin Lee, Mijeong Ko, Seontae Kim, Dosang Lim, Gemma Park, Sang-Eun Lee
Osong Public Health and Research Perspectives.2023; 14(4): 263. CrossRef


 Cite
Cite