Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 11(5); 2020 > Article
-
Original Article
Immunological Profile and Bacterial Drug Resistance in Pregnant Women: A Cross Sectional Study - Ornella JT Ngalani, Wiliane JT Marbou, Armelle Tsafack Mbaveng, Victor Kuete
-
Osong Public Health and Research Perspectives 2020;11(5):319-326.
DOI: https://doi.org/10.24171/j.phrp.2020.11.5.08
Published online: October 22, 2020
Department of Biochemistry, Faculty of Science, University of Dschang, Dschang, Cameroon
- *Corresponding author: Victor Kuete, Department of Biochemistry, Faculty of Science, University of Dschang, Dschang, Cameroon, E-mail: kuetevictor@yahoo.fr
Copyright ©2020, Korea Centers for Disease Control and Prevention
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- This study aimed to investigate the immunological and bacterial profiles in pregnant women of Bafang-Cameroon.
-
Methods
- Stool and midstream urine were cultured using specific culture media. The disk diffusion method was used for the antimicrobial susceptibility test. T-cell lymphocyte counts (CD3, CD4 and CD8), white blood cell counts, sensitive C-reactive protein, and interleukin-6, were measured by flow cytometry, optical detection, and the enzyme-linked immunosorbent assay solid phase direct sandwich method.
-
Results
- Out of 700 participants, 71.43% were pregnant, and 28.57% were non-pregnant women. The mean age was 29.40 ± 8.27 and 27.41 ± 6.55 years in non-pregnant and pregnant women, respectively. CD4 T-cells were not significantly lower in pregnant women compared with non-pregnant women. There were 43.65% and 56.35% bacteria isolates obtained from urine and stool samples, respectively. Bacteria were mostly isolated in patients with a CD4 T-cell count between 461 and 806 cells/μL. Isolates of Klebsiella pneumoniae and Enterobacter aerogenes showed 100% resistance in non-pregnant women, however all isolated bacteria were shown to be multidrug resistant in pregnant women. Salmonella sp. (24.3%) and Escherichia coli (21.51%) showed an increase in multidrug resistant phenotypes in pregnant women.
-
Conclusion
- This study demonstrated that routine bacteriological analysis during pregnancy is necessary for their follow-up care.
- Bacterial infections involving a variety of pathogens like Escherichia coli, Salmonella sp., Shigellla sp., Yersinia enterocolitica, Vibrio parahaemolyticus [1] can occur during pregnancy. Such infections are associated with an increased risk of miscarriage, stillbirth or neonatal death [2]. In pregnant women, bacterial infections can alter the implantation of the fertilized ovum during consignment and peripartum periods, or affect the fetus as well as the new-born [3]. Many women with these infections are asymptomatic, which requires both a high level of clinical awareness and adequate screening. This situation is much more complicated when multidrug resistant bacteria are involved during pregnancy [4].
- Bacterial resistance to antimicrobial agents during pregnancy is an increasing health concern [5]. Pregnancy causes numerous changes in a woman’s body. During pregnancy, the body’s immune system is reduced. Hormonal and mechanical changes increase the risk of urinary stasis and vesicoureteral reflux. These changes, along with an already shortened urethra and difficulties maintaining strict hygienic conditions due to a distended pregnant belly, increase the frequency of urinary tract infections in pregnant women [6]. There is a decrease in bacterial susceptibility to almost all available antibiotics [7]. The 2013 report of United States Centers for Disease Control and prevention declared that at least 2 million people in the USA acquired infections with resistant bacterial phenotypes, with at least 23,000 people dying yearly because of these infections [8]. It was demonstrated in 2014 that, 7.29 % (n = 482,917) of women received outpatient care for urinary tract infections during the 90 days before the date of their last menstrual period or during pregnancy [9]. Given the potential risks associated with the use of some of these antibiotics in early pregnancy and the potential unrecognized pregnancy, women’s healthcare providers should be familiar with the American College of Obstetricians and Gynecologists recommendations and consider the possibility of early pregnancy when treating women of reproductive age [10].
- In Africa in general, although controlling the spread of resistant bacteria is a priority, an update to determine the extent of the resistance phenomenon in pregnant women in various regions is necessary. The objective of this work was to study the hematological and epidemiological profiles of pathogenic bacteria from the enteric and urinary tracts of pregnant women in the town of Bafang, West Region of Cameroon, while also determining the extent of antibiotic resistance during pregnancy.
Introduction
- 1. Study framework
- This was a cross-sectional epidemiological study carried out over a period of 3 years (from November 2016 to September 2019) in pregnant women (without distinction of gestational age of their fetus) who attended antenatal consultation in health centers in Bafang (Western Region of Cameroon) including Adlucem Banka Bafang, District Hospital, Dokovie Bafang Annex Centre. Pregnant women with hepatitis or HIV were not included in this study. Non-pregnant women who did not have HIV or hepatitis were also included in the study.
- 2. Biological material
- Urine, blood, and stool samples were collected from 700 voluntary patients (500 patients were pregnant women, and 200 were not pregnant) who were hospitalized or receiving consultations whilst not receiving antibiotic therapy, and had given written informed consent. Sociodemographic information (age, marital status, gestational age, activity carried out, total number of pregnancies) was obtained, and questionnaires were administered. Duplicate questionnaires were systematically eliminated.
- 3. Collection of samples
- In this study, samples (blood, urine, and stool) were collected under aseptic conditions for 700 women and were analyzed within 2 hours. There was 10 mL of blood collected directly into 3 tubes; 2 containing the anticoagulant (EDTA) and 1 tube without anticoagulant. The blood samples were sent directly for analysis of white blood cell counts, C-reactive protein (CRP), interleukin-6 (IL-6), and CD4, CD3, and CD8-T lymphocytes. Stool and urine samples were sent for microbiological analyses.
- 4. Analysis of blood samples
- The blood in the EDTA tube was analyzed after being homogenized (to avoid the formation of blood clots), using a cell counter (Mindray PE 6800, PROCAN, China, Mainland) for the white blood cells which gave the total number of white blood cells, lymphocytes, monocytes, and granulocytes. The counting of CD4, CD3 and CD8 T-lymphocytes was carried out by flow cytometry using a FACS Calibur machine (Becton Dickinson, San Jose, CA, USA). Depending on the number of cells obtained, the cells were stained at room temperature for 30 minutes with 100 mL of anti-CD4 and PE-anti-CD8 monoclonal antibodies labelled with fluorescein isothiocyanate, then washed once in the FACS buffer. The CD4 and CD8 surface expression was visualized using flow cytometry. Lymphocytes were defined by the sideward and forward diffusion parameter, and they were controlled in such a way that cells in the defined window could be taken into consideration. The flow cytometry data were analyzed within 24 hours of staining using software (FlowJo). The results were expressed as percentage of positive flow cytometry for CD4 and CD8. The measurement of IL-6 was performed using Enzyme-Linked Immunosorbent Assay (ELISA) kits (R&D system, London, UK), according to the manufacturer’s instructions. Blood collected in the tube without an anticoagulant was analyzed by spectrophotometry for the quantification of CRP (Actim CRP, the Medix Biochemica Company, Finland). The highly sensitive (hs)-CRP was measured in duplicate by a high sensitivity ELISA, as described above. The sensitivity of the assay was 0.2 ng/mL. The intra and the inter assay variability were measured as 3.9% and 7.4%, respectively.
- 5. Bacterial isolation
- Salmonella-Shigellla agar, Manitol salt agar, Hecktoen agar, Eosin Methylene Blue, Cystine Lactose Electrolyte Deficient, Muller Hinton agar and the MacConkey agar were used for the isolation and phenotypic identification of bacteria. The following antibiotic discs were used: amikacin (AMI, 30 μg), imipenem (IMI, 10 μg), amoxicillin + clavulanic acid (AUG, 20 μg), ciprofloxacin (CIP, 5 μg), norfloxacin (NOR 10 μg), ceftriaxone (CEFT,30 μg), erythromicin (ERY, 15 μg), doxycycline (DOX, 30 μg), vancomycin (VAN, 30 μg) chloramphenicol (CHL, 30 μg) amoxicillin (AMO, 10 μg), cefuroxin (CEFU, 30 μg), gentamicin (GEN, 30 μg), tetracycline (TET, 30 μg), nitrofurantoin (NIT, 100 μg), ofloxacin (OFL, 5 μg), and sulfamethoxazole + trimethoprim (SUL, 25 μg). Super cooled agar culture media were poured into tri-segmented petri dishes (90 mm). After the solidification of the culture media at room temperature, the liquid stool samples were directly streaked into the culture media, while the urine samples were flooded onto the culture media, and incubated at 37°C for 24 hours. Solid stools were initially dissolved in sterile physiological water. The bacterial colonies of the stool observed on the surface of the culture media after incubation, were purified by successive sub-culturing until pure colonies or clinical isolates were obtained. The conventional method of interpretation of bacterial colonies of urine (criteria defined by Kass) was used and the parameters of the urinary infection such as leukocyturia > 104/mL, and bacteriuria in colony forming unit (CFU) per mL > 105 per mL (threshold of E coli was lowered to 103 CFU/mL and considered to be uropathogenic) were taken into consideration. After macroscopic observation of the colonies, the identification of bacteria, based on the study of biochemical and enzymatic characters, was performed using the API 20E galleries (bio Mérieux SA, Marcy L'Etoile, France).
- 6. Antibiotic susceptibility test
- The susceptibility study of the different isolates was carried out using the Mueller-Hinton agar diffusion technique according to the protocol recommended by the Clinical and Laboratory Standard Institute [11] with some modifications. A volume of 2 mL of bacterial suspension (1.5 × 106 CFU/mL) was spread on the surface of a 90 mm Petri dish previously cast with Mueller-Hinton agar. This was then placed in a hot air oven for 5 minutes and the antibiotic discs selected accordingly. Clinical isolates were placed on the surface of the agar using sterile forceps. After 15 minutes, the petri dish was incubated for 24 hours at 37°C, and the interpretation was made according to the recommendations of the Antibiogram Committee of the French Society of Microbiology (CA-SFM, 2018), followed by measurement of the diameters of the inhibition zones using a graduated ruler.
- 7. Ethical approval
- Ethics approval was granted by the Ethics Review and Advisory Committee of CAMBIN for this study on the 24th April 2019 (CBI/437/ERCC/CAMBIN) and approval from the director of the Bafang Health Area was granted. Information sheets detailing the purpose and process of the study were provided to each participant. Participants gave written and informed consent for voluntary participation.
- 8. Processing and statistical analysis of data
- In establishing the percentages of resistance of the different bacterial species, the results were described as "intermediate," "resistant," and "susceptible." Descriptive analysis of the data was performed using SPSS Version 20 (IBM Corp., Armonk, NY, USA) and Microsoft Excel 2010. Proportions were compared using the chi-square test. Student t test was used to compare continues variables. A value of p < 0.05 was considered statistically significant.
Materials and Methods
- There were 700 patients studied, 500 (71.43%) were pregnant women and 200 (28.57%) were non-pregnant women who gave stool and/or urine samples for analysis. These patients included women who were in a relationship (married, cohabiting) or single (single, separated, divorced, widowed). Table 1 shows that 324 (73.80%) pregnant women were in a relationship compared with 115 (26.20%) non-pregnant women. There were 176 (67.43%) pregnant women who were single, compared with 85 (32.57%) non-pregnant women. There was a statistically significant difference in the distribution of the age groups in the study (p = 0.001). The mean age of the participants was higher in non-pregnant women (29.40 ± 8.265 years) compared with pregnant women (27.41 ± 6.547 years).
- Table 2 shows the mean values of the blood tests obtained in the study population, as well as the white blood cell, CD4, CD3 and CD8 T-lymphocytes, hs-CRP and IL-6 levels. Results show that, CD4 T-cell counts were lower but not significantly lower in pregnant compared with non-pregnant women. Contrarily, total white blood cells (p = 0.782), granulocytes (p = 0.905), CD8 T-cell (p = 0.490) and hs-CRP (p = 0.421) were higher but non-significant in pregnant women compared to those who were non-pregnant. A total of 252 bacterial isolates were obtained in this study, of which 187 (74.20%) and 65 (25.80%) isolates were obtained from pregnant and non-pregnant women, respectively. A total of 110 (43.65%) isolates were obtained from urine samples, whereas 142 (56.35%) isolates were obtained from stool samples.
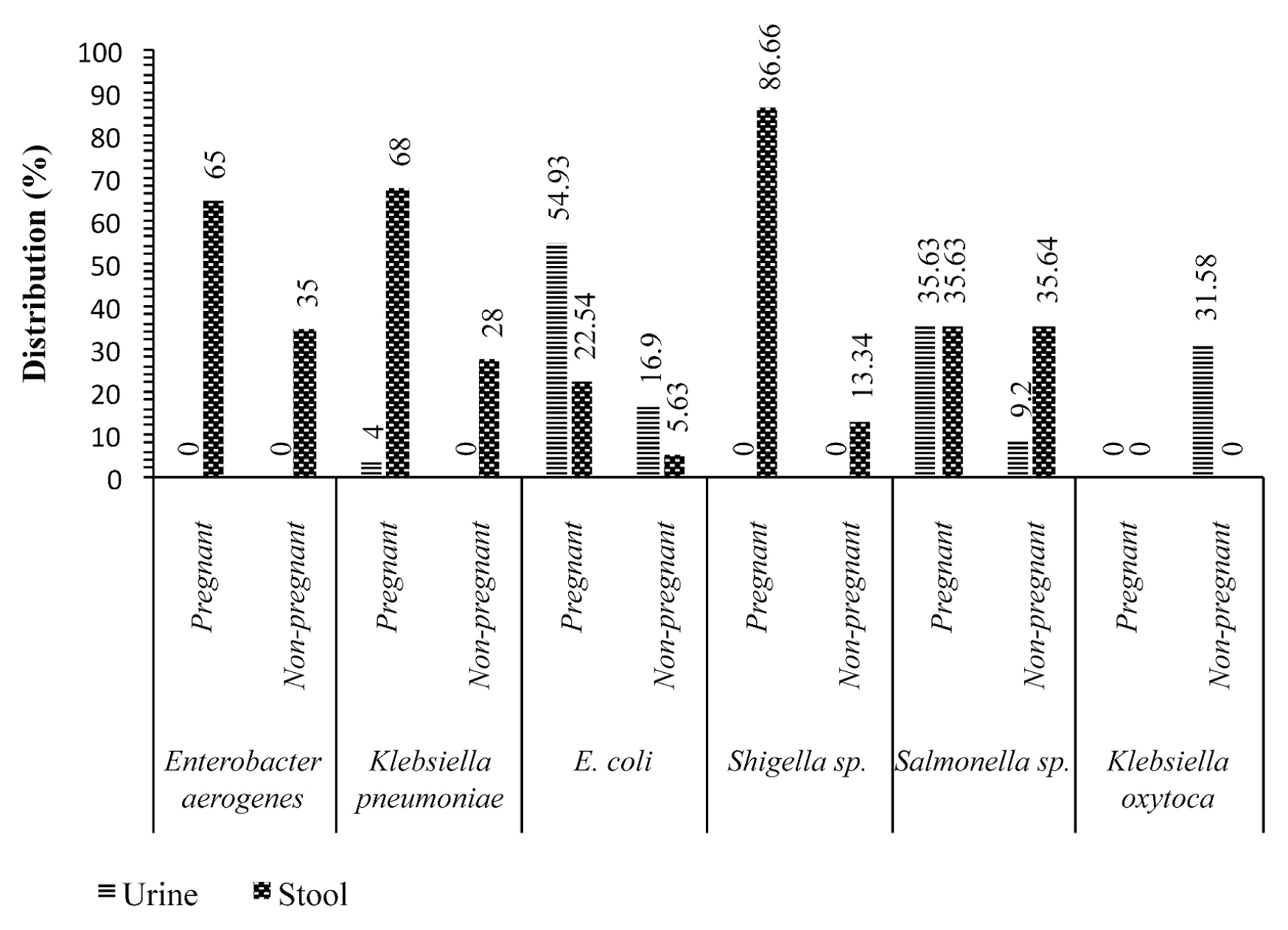
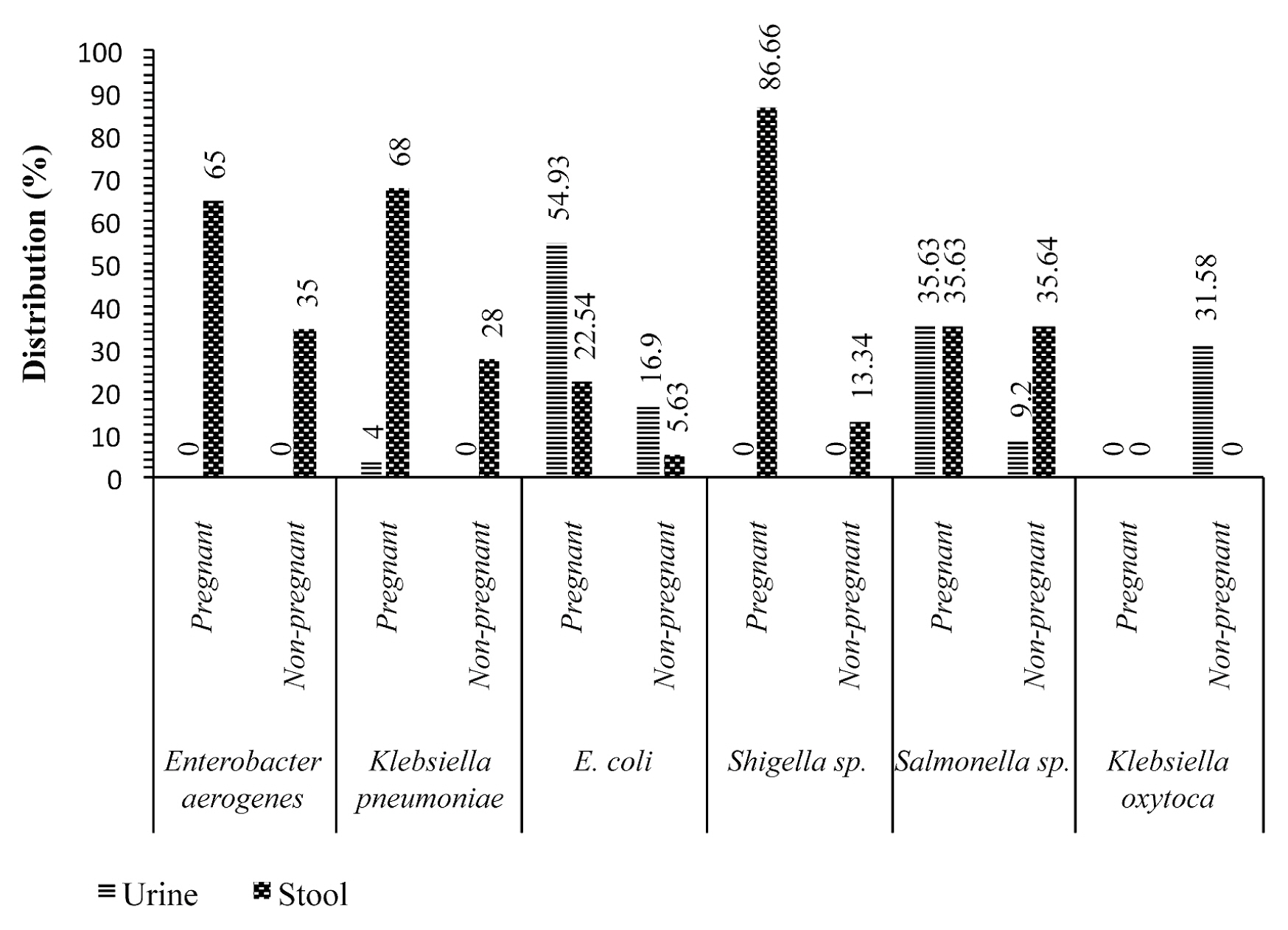
- Figure 1 shows that, Shigellla sp., Klebsiella pneumoniae, Enterobacter aerogenes and Escherichia coli were isolated more frequently in stool samples from pregnant women with values of 26 (86.66%), 17 (68%), 13 (65%), 16 (22.54%), respectively, compared with non-pregnant women with values of 4 (13.34%), 7 (28%), 7 (35%), 4 (5.63%), respectively. On the other hand, Escherichia coli, Salmonella sp. and Klebsiella pneumoniae isolates were identified more in urine samples from pregnant women with 39 (54.93%), 31 (35.63%) and 1 (4%), compared with non-pregnant women with 12 (16.9%), 8 (9.2%) and 0%, respectively.
- Table 3 shows the frequency of bacteria isolates and their association with different blood parameter intervals. It is seen that bacteria was isolated more frequently in patients with a CD4 T-cell count between 461 and 806 cells/μL. Isolates of Shigellla sp. were higher (69.23%) in pregnant women with a CD4 T-cell count between 114–460 cells/μL and p value of 0.317. In contrast, in CD3 and CD8 T-lymphocytes, Shigellla sp. isolates were higher, that is, between 569–1,045 (46.15%) and 10–277 (46.15%) respectively. However, more bacteria were isolated from patients with serum IL-6 levels 25–230 (pg/mL). Salmonella sp. isolates were also higher (95.16%) but not significantly, in pregnant women with serum IL-6 levels 25–230 (pg/mL).
- The susceptibility of the isolates obtained from 14 different antibiotics was assessed in this study. Table 4 shows the susceptibility results of the isolates to these antibiotics. It can be seen that Salmonella sp. and E coli were susceptible to IPM (51.61%), (78.18%); CIP (59.68%), (70.9%); CHL (46.77%), (81.82%); AMO (72.58%), (54.55%); TET (69.35%), (74.55%); AUG (53.23%), (87.27%); and CEFT (56.45%), (76.36%); respectively, in pregnant women. On the other hand, isolates of Klebsiella pneumoniae and Enterobacter aerogenes showed 100% resistance in non-pregnant women.
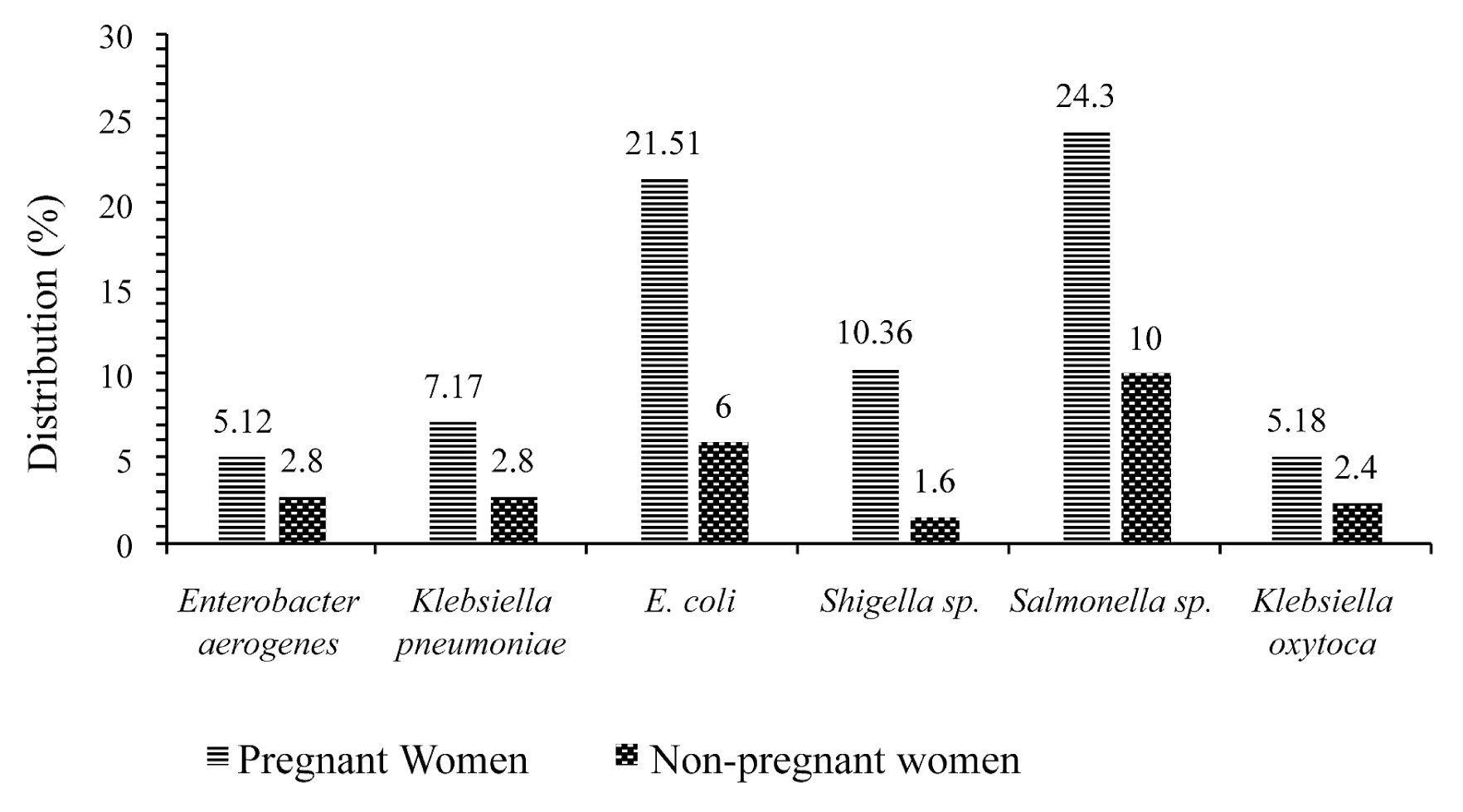
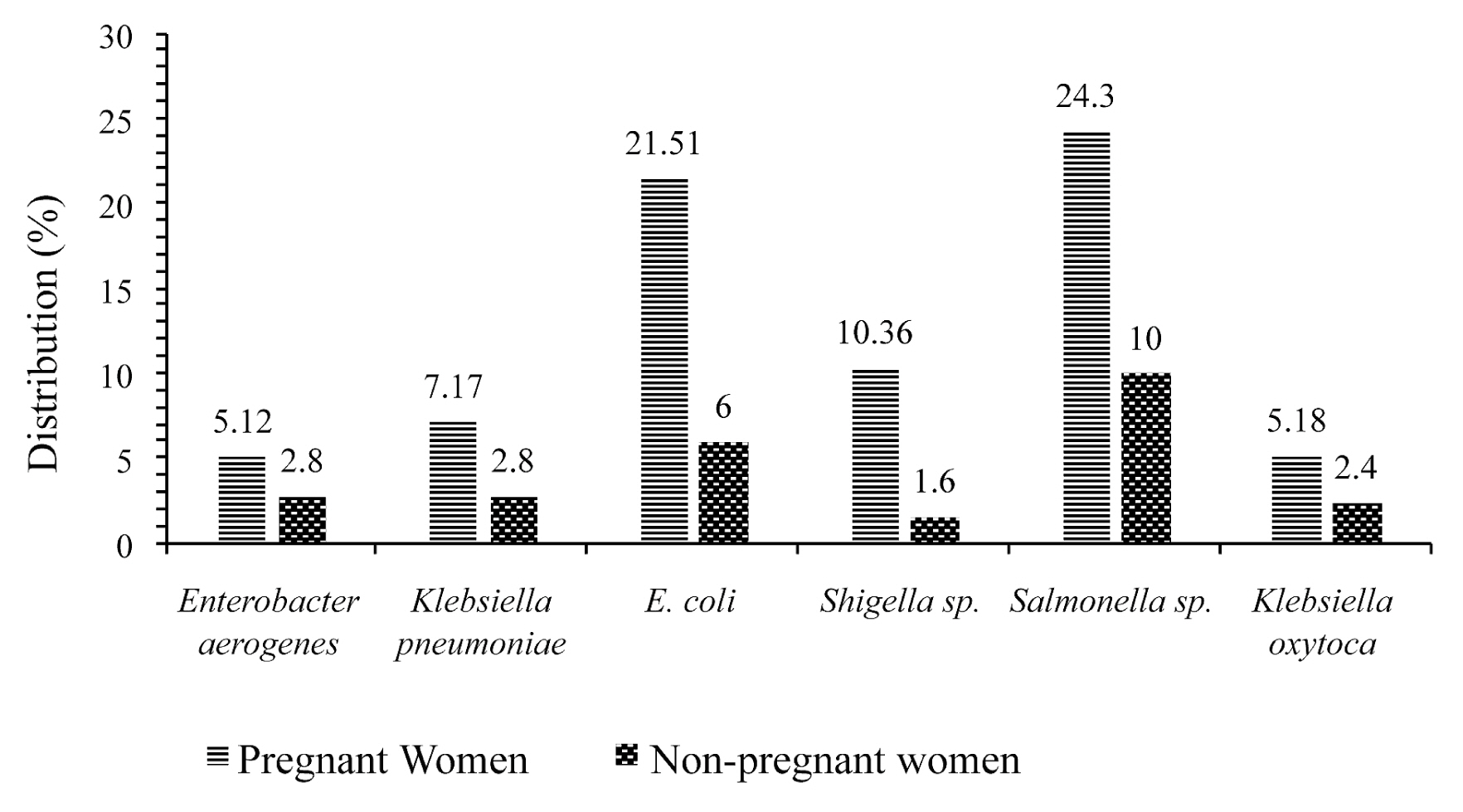
- Figure 2 shows the frequency of multidrug resistance of different isolates in pregnant and non-pregnant women. All bacteria show multidrug resistance in pregnant women compared to non-pregnant women. It can be seen that isolates of Salmonella sp. (24.3%) and Escherichia coli (21.51%) showed an increase multidrug resistance in pregnant women.
Results
- Pregnancy increases the protentional incidence of many bacterial and viral diseases by suppressing immunity. In turn this increased susceptibility may lead miscarriages caused by infections or birth defects in the fetus [10]. Pregnancy is a state in which the immune system does not mount an immune response against foreign paternal antigens of the fetus, but needs to protect the mother and the fetus from invading pathogens. In the present study, most pregnant women were in the age groups 25–34 (74.25%), and 14–24 (74.26%). This result was similar to the statistics reported by Essiben et al in 2017 [12] with an estimated pregnancy prevalence of 31.8% in 20–25 year old’s in 2019. The mean pregnancy age in this current study was 27.41 ± 6.547 years, similar to that observed by Frauke et al in 2009 [13].
- The high average age of clinical presentation of pregnancy in Bafang, West Cameroon can be explained by the culture of family values that prepare young boys and girls to become responsible adults, through various rituals, and close family members who serve as guardians who prohibit premarital sex. This is especially the case for women, and ancestral doctrines discourage parental discussions with children, especially on sex issues, which greatly influences attitudes and behaviors [14].
- It was observed that CD4 T-cell counts were lower, but not statistically significantly lower, in pregnant compared with non-pregnant women. In addition, levels of total white blood cells (p = 0.782), granulocytes (p = 0.905), CD8 (p = 0.490) and CRP (p = 0.421) were higher, but not statistically significantly higher, in pregnant women compared with those who were non-pregnant. These immune system cells are involved in protecting the body against infectious diseases and foreign invaders [15]. Disorders of the hematopoietic system are common throughout pregnancy [16]. In addition, the increase in the number of white blood cells during pregnancy may not always indicate an infection [16]. The absolute value of CD4 T-cells was 499.46 ± 181,807 cells/μL, which was lower than those previously reported. Babatope et al [17] reported a mean value of 614.49 cells/μL in pregnant patients residing in Ekpoma, Nigeria, and Tanjong et al [18] reported a mean value of 851 cells/μL in pregnant women residing in Buea, Cameroon. These higher figures may be due to physiological leukocytosis resulting from repeated infections.
- The etiology of enteric and/or urinary disorders in pregnant women are multifactorial. Stool and urine samples were examined in this study. Bacteria were isolated from 187 (37.4%) pregnant women and 65 (32.5%) non-pregnant women. The high isolation frequency in pregnant women than non-pregnant women may be linked to pregnancy, which weakens the defense mechanisms in pregnant women [6]. Shigella sp, Klebsiella pneumoniae, Enterobacter aerogenes and Escherichia coli were isolated more frequently in stool samples of pregnant women with values 26 (86.66%), 17 (68%), 13 (65%), 16 (22.54%), respectively, than compared with non-pregnant women whose values were 4 (13.34%), 7 (28%), 7 (35%), 4 (5.63%). On the other hand, Escherichia coli, Salmonella sp. and Klebsiella pneumoniae isolates were more frequently isolated in urine samples from pregnant women with 39 (54.93%), 31 (35.63%) and 1 (4%), respectively, compared with non-pregnant women whose values were 12 (16.9%), 8 (9.2%) and 0%, respectively. These bacteria are among those that cause various pregnancy-related infections [19,20].
- The isolation of the bacteria and their association with CD4 T-cell counts, serum hs-CRP and IL-6 levels were studied. In the present study, bacteria isolated from patients with CD4 T-cell counts between 461 and 806 cells/μL showed the relationship between CD4 T-cell count and the presence of bacteria in pregnant women was not significant. Isolates of Shigella sp. were higher (69.23%) in pregnant women with a CD4 T-cell count between 114–460 cells/μL but this was not significantly different to the number of isolates in non-pregnant women with the same CD4 T-cell count (p = 0.317). In CD3 and CD8 T-lymphocytes, Shigella sp. isolates were more frequently observed between 569–1,045 cells/μL (46.15%) and 10–277 cells/μL (46.15%). Bacteria were more frequently isolated from patients with serum IL-6 levels of 25–230 pg/mL. Salmonella sp. isolates were more frequently isolated (95.16%) in pregnant women with serum IL-6 levels 25–230 pg/mL but this observation was not significant. IL-6 is a multifunctional cytokine that plays a key role in the inflammatory response and in the direction of T-cell differentiation in adaptive immunity [21]. IL-6 is widely expressed in the female reproductive tract and gestational tissues thus, exerting regulatory functions in embryonic implantation and placental development, as well as the immune adaptations necessary to tolerate pregnancy [22].
- Results of antimicrobial susceptibility testing revealed that, most isolates of Salmonella sp. and E. coli were more susceptible to IPM (51.61%), (78.18%); IPC (59.68%), (70.9%); CHL (46.77%), (81.82%); AMO (72.58%), (54.55%); TET (69.35%), (74.55%); AUG (53.23%), (87.27%) and CEFT (56.45%), (76.36%) respectively, in pregnant women. Isolates of Klebsiella pneumoniae and Enterobacter aerogenes showed 100% resistance in non-pregnant women. However, all bacteria showed multidrug resistance in pregnant women. Salmonella sp. (24.3%) and Escherichia coli (21.51%) showed multidrug resistance in pregnant women. This resistance may be a result of the high use of antibiotics by pregnant women [23].
- This study was designed to understand the burden of bacterial disease in pregnancy. The representation of these findings in the entire pregnant and non-pregnant women population in the studied area was limited due to the number of participants. The cross-sectional nature of the study does not allow deduction of a cause and effect relationship between parameters and presumed etiological factors.
Discussion
- This study documented the hematological profile and the resistance of pathogenic bacteria to antibiotics in pregnant women. The results of antimicrobial susceptibility testing revealed that most isolates of Salmonella sp. and E. coli were more susceptible to IPM, CIP, CHL, AMO, TET, AUG and CEFT in both pregnant and non-pregnant women, whereas Klebsiella pneumoniae and Enterobacter aerogenes showed a high resistance profile in both populations. All bacteria showed multidrug resistance in pregnant compared with non-pregnant women. Salmonella sp. and Escherichia coli showed an increase multidrug resistance in pregnant women. Pathogenic bacteria were more frequently isolated in participants with a CD4 T-cell count between 461 and 806 cells/μL. Routine bacteriological analyses during pregnancy are recommended for prompt attention and treatment to avoid complications.
Conclusion
-
Acknowledgements
- The authors would like to thank directors and staff of Banka Adlucem Hospital Bafang, Bafang District Hospital, and Dokovie Bafang Annex Centre. Special acknowledgement goes to patients whom accepted the invite to participate in this study and the entire general hospital laboratory personnel.
Acknowledgments
-
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Article information
Supplementary Materials
- 1. Mendz GL, Kaakoush NO, Quinlivan JA. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front Cell Infect Microbiol 2013;3:58PMID: 10.3389/fcimb.2013.00058. PMID: 24137568. PMID: 3797391.ArticlePubMedPMC
- 2. McClure EM, Dudley DJ, Reddy UM, et al. Infectious causes of stillbirth: A clinical perspective. Clin Obstet Gynaecol 2010;53(3). 635−45. PMID: 10.1097/GRF.0b013e3181eb6620.Article
- 3. Jeffery HE, Lahra MM. The Impact of Infection During Pregnancy on the Mother and Baby Fetal and Neonatal Pathology. London (UK): Springer London; 2007. pp 379−423. PMID: 10.1007/978-1-84628-743-5_16.
- 4. Leeper C, Lutzkanin A. Infections During Pregnancy. Prim Care 2018;45(3). 567−86. PMID: 10.1016/j.pop.2018.05.013. PMID: 30115342.ArticlePubMed
- 5. Khan S, Rashmi , Singh P, et al. Pregnancy-associated asymptomatic bacteriuria and drug resistance. J Taibah Univ Med Sci 2015;10(3). 340−5.Article
- 6. Ovalle A, Levancini M. Urinary tract infections in pregnancy. Curr Opin Urol 2001;11(1). 55−9. PMID: 10.1097/00042307-200101000-00008. PMID: 11148747.ArticlePubMed
- 7. Karam GH, Heffner JE. Emerging issues in antibiotic resistance in blood-borne infections. Am J Respir Crit Care Med 2000;162(5). 1610−6. PMID: 10.1164/ajrccm.162.5.pc10-00. PMID: 11069784.ArticlePubMed
- 8. Al Rahmany D, Albeloushi A, Alreesi I, et al. Exploring bacterial resistance in Northern Oman, a foundation for implementing evidence-based antimicrobial stewardship program. Int J Infect Dis 2019;83:77−82. PMID: 10.1016/j.ijid.2019.04.004. PMID: 30959249.ArticlePubMed
- 9. Ailes EC, Summers AD, Tran EL, et al. Antibiotics dispensed to privately insured pregnant women with urinary tract infections - United States, 2014. Morb Mortal Wkly Rep 2018;67(1). 18−22. PMID: 10.15585/mmwr.mm6701a4.Article
- 10. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee opinion no. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol 2011;117(6). 1484−5. PMID: 10.1097/AOG.0b013e3182238c57. PMID: 21606771.ArticlePubMed
- 11. Clinical and Laboratory Standards Institute. Performance standard for antimicrobial susceptibility testing. 27th Ed. CLSI Supplement M100. Wayne (PA): Clinical and Laboratory Standards Institute; 2017.
- 12. Félix E, Yemga DVW, Um Meka EN, et al. Eclampsia in African Milieu, Yaounde-Cameroon: epidemiology, seasonal variations and treatment regimen. Obstet Gynecol Int J 2019;10(3). 176−83.Article
- 13. Von Versen-Hoeynck FM, Hubel CA, Gallaher MJ, et al. Plasma levels of inflammatory markers neopterin, sialic acid, and C-reactive protein in pregnancy and preeclampsia. Am J Hypertens 2009;22(6). 687−92. PMID: 10.1038/ajh.2009.54. PMID: 19282816. PMID: 2737463.ArticlePubMedPMCPDF
- 14. Bahoken JC, Jean C, Atangana E. Cultural policy in the United Republic of Cameroon. Unesco; 1976.
- 15. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol 2010;125(2). S3−23. PMID: 10.1016/j.jaci.2009.12.980. PMID: 20176265. PMID: 2923430.ArticlePubMedPMC
- 16. Townsley DM. Hematologic complications of pregnancy. Semin Hematol 2013;50(3). 222−31. PMID: 10.1053/j.seminhematol.2013.06.004. PMID: 23953339. PMID: 3748382.ArticlePubMedPMC
- 17. Babatope IO, Isabu PA, Imarenezor EPK, et al. Normal CD4, CD8 T-lymphocytes and leucocyte baseline in healthy HIV-seronegative pregnant women in Ekpoma, Edo State, Nigeria. Int J Basic Appl Innovat Res 2018;7(1). 18−28.
- 18. Tanjong R, Atashili J, Kamga H, et al. Reference Values of CD4-Lymphocyte Counts in HIV Seronegative Pregnant Women in Buea, Cameroon. African J Clin Exp Microbiol 2011;13(1). 28−33.Article
- 19. Gravett CA, Gravett MG, Martin ET, et al. Serious and Life-Threatening Pregnancy-Related Infections: Opportunities to Reduce the Global Burden. PLoS Med 2012;9(10). e1001324PMID: 10.1371/journal.pmed.1001324. PMID: 23055837. PMID: 3467240.ArticlePubMedPMC
- 20. Ndamason LM, Marbou WJT, Kuete V. Urinary tract infections, bacterial resistance and immunological status : a cross sectional study in pregnant and non-pregnant women at Mbouda Ad-Lucem Hospital. Afri Health Sci 2019;19(1). 1525−35. PMID: 10.4314/ahs.v19i1.26.Article
- 21. Choy E, Rose-John S. Interleukin-6 as a Multifunctional Regulator: Inflammation, Immune Response, and Fibrosis. J Scleroderma Relat Disord 2017;2(Suppl 2). S1−5. PMID: 10.5301/jsrd.5000265.Article
- 22. Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol 2012;95(1–2). 1−14. PMID: 10.1016/j.jri.2012.05.004. PMID: 22819759.ArticlePubMed
- 23. De-Tejada BM. Antibiotic use and misuse during pregnancy and delivery: Benefits and risks. Int J Environ Res Public Health 2014;11(8). 7993−8009. PMID: 10.3390/ijerph110807993.ArticlePubMedPMC
References
a = pregnant women; AMI = amikacin; AMO = amoxicillin; AUG = Amoxicillin + clavulanic acid; b = Non-pregnant women; CEFT = ceftriaxone; CEFU = cefuroxin; CHL = chloramphenicol; CIP = ciprofloxacin; DOX = doxycycline; ERY = erythromycin; GEN = gentamicin; I = intermediate; IMI = imipenem; NIT = nitrofurantoin; frequency (%); OR = norfloxacin; R = resistant; s = susceptible; TET = tetracycline; VAN = vancomycin.
Figure & Data
References
Citations

- Methanol extract from the seeds of Persea americana displays antibacterial and wound healing activities in rat model
Steve E. Ekom, Jean-De-Dieu Tamokou, Victor Kuete
Journal of Ethnopharmacology.2022; 282: 114573. CrossRef - Antibacterial and Therapeutic Potentials of the Capsicum annuum Extract against Infected Wound in a Rat Model with Its Mechanisms of Antibacterial Action
Steve Endeguele Ekom, Jean-De-Dieu Tamokou, Victor Kuete, Dorota Formanowicz
BioMed Research International.2021; 2021: 1. CrossRef


 PubReader
PubReader ePub Link
ePub Link Cite
Cite