Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 6(4); 2015 > Article
-
Original Article
Expression of Recombinant pET22b-LysK-Cysteine/Histidine-Dependent Amidohydrolase/Peptidase Bacteriophage Therapeutic Protein inEscherichia coli BL21 (DE3) - Hamed Haddad Kashani, Rezvan Moniri
-
Osong Public Health and Research Perspectives 2015;6(4):256-260.
DOI: https://doi.org/10.1016/j.phrp.2015.08.001
Published online: August 21, 2015
Anatomical Sciences Research Center, Kashan University of Medical Sciences, Kashan, Iran
- ∗Corresponding author. Moniri@kaumsc.ac.ir
• Received: January 14, 2015 • Revised: August 2, 2015 • Accepted: August 6, 2015
Copyright © 2015 Korea Centers for Disease Control and Prevention. Published by Elsevier Korea LLC. All rights reserved.
Abstract
-
Objectives
- Bacteriophage-encoded endolysins are a group of enzymes that act by digesting the peptidoglycan of bacterial cell walls. LysK has been reported to lyse live staphylococcal cultures. LysK proteins containing only the cysteine/histidine-dependent amidohydrolase/peptidase (CHAP) domain has the capability to show lytic activity against live clinical staphylococcal isolates, including methicillin-resistant Staphylococcus aureus (MRSA). The aim of this study was to clone and express LysK-CHAP domain in Escherichia coli BL21 (DE3) using pET22b as a secretion vector. The pET22b plasmid was used, which encoded a pelB secretion signal under the control of the strong bacteriophage T7 promoter.
-
Methods
- The E. coli cloning strains DH5α and BL21 (DE3) were grown at 37°C with aeration in the Luria-Bertani medium. A plasmid encoding LysK-CHAP in a pET22b backbone was constructed. The pET22b vector containing LysK-CHAP sequences were digested with NcoI and HindIII restriction enzymes. Cloning accuracy was confirmed by electrophoresis. The pET22b-LysK plasmid was used to transform the E. coli strain BL21. Isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to a final concentration of 1mM to induce T7 RNA polymerase-based expression. Finally, western blot confirmed the expression of target protein.
-
Results
- In this study, after double digestion of pEX and pET22b vectors with HindIII and NcoI, LysK gene was cloned into two HindIII and NcoI sites in pET22b vector, and then transformed to E. coli DH5α. Cloning was confirmed with double digestion and analyzed with agarose gel. The recombinant pET22b-LysK plasmid was transformed to E. coli BL21 and the expression was induced by IPTG. The expression was confirmed by Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting method. Observation of a 28.5 kDa band confirmed LysK protein expression.
-
Conclusion
- In the present study, LysK-CHAP domain was successfully cloned and expressed at the pET22b vector and E. coli BL21 (DE3).
- Lysins are phage-encoded peptidoglycan hydrolases, which, when applied exogenously as purified recombinant proteins to Gram-positive bacteria, bring about rapid lysis and death of the bacterial cell 1, 2. Unlike antibiotics, phage lysins can be used to selectively target specific pathogenic bacteria without affecting the surrounding commensal microflora. They are reported to have a narrow host range similar to that of their phage producers, rendering them generally either species 3, 4 or genus specific 5, 6. The potential of these enzymes as novel therapeutics against a number of Gram-positive pathogens on mucosal surfaces and in systemic infections has previously been demonstrated 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. In relation to bacterial resistance, no strains have yet been identified with resistance to phage lysins 1, 3, 14. It has been suggested that these enzymes have evolved to target specific molecules in the host peptidoglycan that are essential for cell viability, making resistance an unlikely event 17, 18. In general, Staphylococcus aureus phage lysins display a multidomain modular structure comprising a C-terminal cell wall binding domain and two N-terminal catalytic domains. Examples include LysK, MV-L, phi11, and LysH5 15, 19, 20. Native lysins of S. aureus, with the exception of MV-L, have typically shown poor expression, insolubility, and low activity when generated as recombinant proteins in a heterologous host 21, 22, 23.
- However, a number of lysins have been reported, which consists of two catalytic domains including those of S. aureus (LysK, Phi11, and MV-L), where endopeptidase activity is a common feature 5, 15, 24. Of all lysins reported to date, the streptococcal phage lysin PlyC is particularly unique, as it displays a multimeric modular structure consisting of two distinct gene products designated PlyCA (50 kDa heavy chain) and PlyCB (8 kDa light chain) [25]. The C-terminal binding domain of the majority of lysins is responsible for attaching the enzyme to its specific substrate in the bacterial cell wall via noncovalent binding of carbohydrate ligands [26]. A recent study on the crystal structure of the pneumococcal phage lysin Cpl-1 in free and choline-bound states suggested that the choline-binding domain assists in the correct positioning of the N-terminal catalytic domain [27]. Although it appears that the C-terminal domain is necessary for lytic activity of some endolysins 14, 23, 26, this is not always the case. A number of enzymes have shown an increased lytic activity upon removal of the binding domain 28, 29, 30. For example, when LysK was truncated to its N-terminal endopeptidase domain, cysteine/histidine-dependent amidohydrolase/peptidase (CHAP), it had a two-fold higher lytic activity than the native enzyme [30]. It is possible that the C-terminal binding domain in the native enzyme may be limiting the potential activity of the N-terminal lytic domain by only allowing it to configure and function when bound to its target in the cell wall 30, 31. In contrast to lysins against Gram-positive pathogens, the enzymes associated with Gram-negative phages are often globular single-module enzymes as in the T7 lysin (lysozyme) 32, 33. LysK has been reported to lyse live staphylococcal cultures. LysK proteins containing only the CHAP domain has the capability to show lytic activity against live clinical staphylococcal isolates, including methicillin-resistant S. aureus (MRSA). The aim of this study is to clone and express LysK-CHAP domain in Escherichia coli BL21 (DE3) using pET22b. In the current research, we used pET22b because particular interest for the expression of disulfide bonded proteins is a family of pET vectors containing the N-terminal pelB secretion signal, which directs synthesized polypeptides to the E. coli periplasm [34]. Disulfide oxidoreductases and isomerases located in the E. coli periplasm catalyze the formation of disulfide bonds, enabling the accumulation of properly folded soluble protein and making the periplasm an ideal compartment for expression of certain therapeutic proteins. [35]. Construction of a single-domain protein for therapeutic purposes is desirable as well as facilitating protein production; this may decrease the probability of a significant immunogenic response. In addition to the main study, we would like to assess extracellular secretion property of pET22b in the Luria-Bertani (LB) medium.
Introduction
- The E. coli cloning strains DH5α and BL21 (DE3) were grown at 37°C with aeration in the LB medium. When appropriate, the LB medium was supplemented with ampicillin (100 μg/mL) for plasmid selection. The growth media for these bacteria were purchased from Himedia Company, Mumbai - India. A plasmid encoding the C-terminally 6xHis-tagged recombinant protein LysK-CHAP in a pEX backbone (MWG-Biotech, Ebersberg, Germany) was constructed. Overexpression of proteins was performed in E. coli BL21 (DE3) (Invitrogen, Carlsbad, CA, USA) cultured at 37°C in modified LB medium (15 g/L tryptone, 8 g/L yeast extract, and 5 g/L NaCl) [36] supplemented with 100 μg/mL ampicillin for plasmid selection and maintenance. Vectors, pEX and pET22b, containing LysK-CHAP sequences were digested with NcoI and HindIII restriction enzymes, and then the sticky vectors were ligated by T4 ligase enzyme. Cloning was confirmed by double digestion. Cloning accuracy was confirmed by electrophoresis.
- 2.1 Protein expression
- The pET22b-LysK-CHAP plasmid was used to transform the E. coli strain BL21, which allows IPTG-regulated expression of T7 RNA polymerase. For confirming the expression, 5 mL cultures were grown in an air shaker (250 rpm) at 37°C in the LB medium containing 100 mg/mL of ampicillin. At OD600 = 0.5, IPTG was added to a final concentration of 1mM to induce the T7 RNA polymerase-based expression. IPTG induction was done at a lower temperature (30°C), which has been shown to enhance LysK protein expression. Finally, western blot confirmed the expression of target protein.
Materials and methods
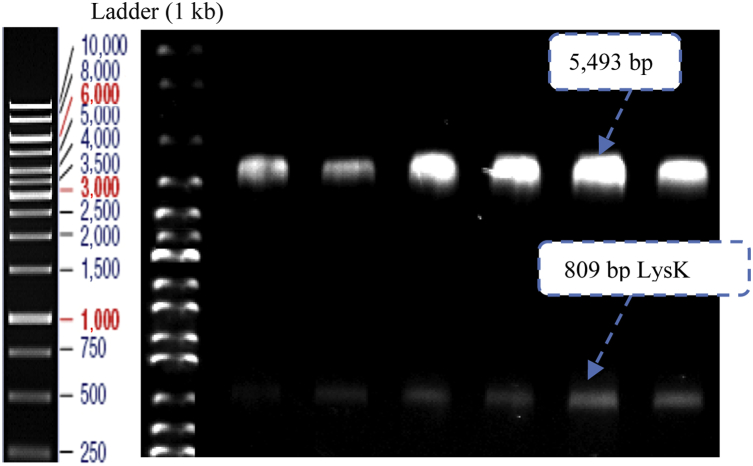
- In the current study, cloning was confirmed with double digestion and analyzed with agarose gel; the presence of an 809-bp band confirmed cloning of the LysK gene (Figure 1).
- Observation of a 28.5 kDa protein band confirmed LysK-CHAP protein expression (Figure 2).
- Although protein expression was confirmed in this study, we could not detect any protein in the LB medium. This means that most of the protein has been secreted in the preplasmic area or inside of the bacterial cell.
Results
- In the present study, LysK-CHAP domain was successfully cloned and expressed at the pET22b vector and E. coli BL21 (DE3). This kind of lysin was previously studied by several scientists 15, 19, 20, but in our study for assessment of extracellular secretion of protein of LysK-CHAP domain, the domain was cloned in the pET22b vector and transformed into E. coli BL21 (DE3) for expression of the desired protein. According to our mentioned data, protein was expressed by the vector but should be scaled up for optimum production. In this study no sign of protein was detected in the LB medium but Cytoplasmic protein expression was confirmed by SDS-PAGE and western blotting.
- To our knowledge, only a few phage endolysins, such as LysK, phi11, MV-L, and LysH5, have been reported to lyse live staphylococcal cultures 15, 19, 20. LysK has a modular structure similar to the structure of these endolysins, with two catalytic domains, a CHAP domain and a central amidase-2 domain (N-acetylmuramoyl-l-alanine amidase), as well as a C-terminal SH3b cell-binding domain. In the current study, we also synthesized the CHAP domain containing 165 amino acids. According to another study [37], this sequence has a strong antibacterial potential. The potential of these enzymes as novel therapeutics against a number of Gram-positive pathogens on mucosal surfaces and in systemic infections has previously been demonstrated 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. In relation to bacterial resistance, no strains with resistance to phage lysins have yet been identified 1, 3. It has been suggested that these enzymes have evolved to target specific molecules in the host peptidoglycan that are essential for cell viability, making resistance an unlikely event 17, 18. In general, S. aureus phage lysins display a multidomain modular structure comprising a C-terminal cell wall binding domain and two N-terminal catalytic domains. Examples include LysK, MV-L, phi11, and LysH5 5, 15. Native lysins of S. aureus, with the exception of MV-L, have typically shown poor expression, insolubility, and low activity when generated as recombinant proteins in a heterologous host [5]. However, their modular structure has enabled the construction of truncated (CHAP) and chimeric versions of lysins (ClyS and P16-17) 16, 22 to help circumvent these problems. Scientists previously showed that the activity of CHAPK against live S. aureus including MRSA was twofold higher than that of the native enzyme LysK [30]. To date, the multidomain MV-L lysin from phage MR11 and the chimeric two-domain lysin ClyS are the only antistaphylococcal lysins that have been evaluated in vivo 15, 16. In this study, LysK proteins containing only the CHAP domain was expressed. Construction of a single-domain protein for therapeutic purposes is desirable, as well as facilitating protein production; this may decrease the possibility of a significant immunogenic response. Unlike antibiotics, intact endolysins are large proteins that are capable of stimulating a humoral immune response, especially when they are used intravenously. Most endolysins have a modular organization with a conserved N-terminal catalytic domain and a more diverse C-terminal cell wall binding domain 37, 38. However, several S. aureus phages produce endolysins with two catalytic domains at their N terminus, such as those from phages K, f11, and fMR11. These lytic enzymes present a CHAP domain followed by an amidase-2 domain (N-acetylmuramoyl-l-alanine amidase), where the CHAP domain seems to be most effective in inducing lysis 5, 15, 24. The LysK-CHAP domain provides a valuable functional unit for domain-swapping studies. It would be interesting to investigate if a chimeric protein with the LysK-CHAP domain and a different substrate-binding domain would have an altered spectrum of inhibition, since it has been demonstrated that the CHAP domain alone has the same spectrum of inhibition in all the strains tested. Environments such as hospitals and nursing homes with a high number of MRSA infections can benefit considerably from exploitation of the CHAP domain of LysK.
- In the current study, we synthesized LysK-CHAP domain and cloned it to the pET22b as a preplasmic secretion vector that enabling to express a lytic protein. Expression of a lytic protein in the preplasmic area of pET22b promises to be a suitable method for the production of lytic proteins for further applications.
Discussion
- All authors declare no conflicts of interest.
Conflicts of interest
-
Acknowledgements
- This study was funded by the Anatomical Sciences Research Center and the Research Deputy of Kashan University of Medical Sciences, as part of an MPhil dissertation, IR.KAUMS.RES.1394. Grant Nr.91105.
Acknowledgments
-
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Article information
- 1. Loessner M.J.. Bacteriophage endolysins—current state of research and applications. Curr Opin Microbiol 8:2005;480−487. PMID: 15979390.ArticlePubMed
- 2. Fenton M., Ross R.P., McAuliffe O.. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs 1:2010;9−16. PMID: 21327123.ArticlePubMedPMC
- 3. Loeffler J.M., Nelson D., Fischetti V.A.. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2001;2170−2172. PMID: 11739958.ArticlePubMed
- 4. Zimmer M., Vukov N., Scherer S.. The murein hydrolase of the bacteriophage phi3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl Environ Microbiol 68:2002;5311−5317. PMID: 12406719.ArticlePubMedPMC
- 5. O'Flaherty S., Coffey A., Meaney W.. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol 187:2005;7161−7164. PMID: 16199588.ArticlePubMedPMC
- 6. Loessner M.J., Maier S.K., Daubek-Puza H.. Three Bacillus cereus bacteriophage endolysins are unrelated but reveal high homology to cell wall hydrolases from different bacilli. J Bacteriol 179:1997;2845−2851. PMID: 9139898.ArticlePubMedPMC
- 7. Cheng Q., Nelson D., Zhu Z.. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother 49:2005;111−117. PMID: 15616283.ArticlePubMedPMC
- 8. Entenza J.M., Loeffler J.M., Grandgirard D.. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob Agents Chemother 49:2005;4789−4792. PMID: 16251333.ArticlePubMedPMC
- 9. Grandgirard D., Loeffler J.M., Fischetti V.A.. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis 197:2008;1519−1522. PMID: 18471063.ArticlePubMed
- 10. Jado I., Lopez R., Garcia E.. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J Antimicrob Chemother 52:2003;967−973. PMID: 14613958.ArticlePubMed
- 11. Loeffler J.M., Djurkovic S., Fischetti V.A.. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect Immun 71:2003;6199−6204. PMID: 14573637.ArticlePubMedPMC
- 12. McCullers J.A., Karlstrom A., Iverson A.R.. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog 3(3). 2007 Mar;e28PMID: 17381239.ArticlePubMed
- 13. Nelson D., Loomis L., Fischetti V.A.. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci U S A 98:2001;4107−4112. PMID: 11259652.ArticlePubMedPMC
- 14. Schuch R., Nelson D., Fischetti V.A.. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:2002;884−889. PMID: 12192412.ArticlePubMed
- 15. Rashel M., Uchiyama J., Ujihara T.. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage ΦMR11. J Infect Dis 196:2007;1237−1247. PMID: 17955443.ArticlePubMed
- 16. Daniel A., Euler C., Collin M.. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:2010;1603−1612. PMID: 20086153.ArticlePubMedPMC
- 17. Garcia P., Lopez R., Ronda C.. Mechanism of phage induced lysis in pneumococci. J Gen Microbiol 129:1983;479−487. PMID: 6132960.ArticlePubMed
- 18. Fischetti V.A.. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol 300:2010;357−362. PMID: 20452280.ArticlePubMedPMC
- 19. Donovan D.M., Lardeo M., Foster-Frey J.. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbial Lett 265:2006;133−139.Article
- 20. Obeso J.M., Martinez B., Rodriguez A.. Lytic activity of the recombinant staphylococcal bacteriophage phiH5 endolysin active against Staphylococcus aureus in milk. Int J Food Microbiol 128:2008;211−218.
- 21. Becker S.C., Foster-Frey J., Donovan D.M.. The phage K enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbial Lett 287:2008;185−191.Article
- 22. Manoharadas S., Witte A., Blasi U.. Antimicrobial activity of a chimeric enzybiotic towards Staphylococcus aureus. J Biotechnol 139:2009;118−123. PMID: 18940209.ArticlePubMed
- 23. Sass P., Bierbaum G.. Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol 73:2007;347−352. PMID: 17085695.ArticlePubMed
- 24. Navarre W.W., Ton-That H., Faull K.F., Schneewind O.. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a D-alanyl-glycine endopeptidase activity. J Biol Chem 274:1999;15847−15856. PMID: 10336488.ArticlePubMed
- 25. Nelson D., Schuch R., Cahales P.. PlyC: a multimeric bacteriophage lysin. Proc Natl Acad Sci U S A 103:2006;10765−10770. PMID: 16818874.ArticlePubMedPMC
- 26. Loessner M.J., Kramer K., Ebel F.. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high- affinity binding to bacterial cell wall carbohydrates. Mol Microbiol 44:2002;335−349. PMID: 11972774.ArticlePubMed
- 27. Hermoso J.A., Monterroso B., Albert A.. Structural basis for selective recognition of pneumococcal cell wall by modular endolysin from phage Cp-1. Structure 11:2003;1239−1249. PMID: 14527392.ArticlePubMed
- 28. Loessner M.J., Gaeng S., Wendlinger G.. The two-component lysis system of Staphylococcus aureus bacteriophage twort: a large TTG-start holin and an associated amidase endolysin. FEMS Microbiol Lett 162:1998;265−274. PMID: 9627962.ArticlePubMed
- 29. Cheng Q., Fischetti V.A.. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl Microbiol Biotechnol 74:2007;1284−1291. PMID: 17186236.ArticlePubMed
- 30. Horgan M., O'Flynn G., Garry J.. The phage lysin, LysK, can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl Environ Microbiol 75(3). 2009 Feb;872−874. PMID: 19047377.ArticlePubMed
- 31. Low L.Y., Yang C., Perego M.. Structure and lytic activity of a Bacillus anthracis prophage endolysin. J Biol Chem 280:2005;35433−35439. PMID: 16103125.ArticlePubMed
- 32. Briers Y., Schmelcher M., Loessnen M.J.. The high-affinity peptidoglycan binding domain of Pseudomonas phage endolysin KZ144. Biochem Biophys Res Commun 383:2009;187−191. PMID: 19348786.ArticlePubMed
- 33. Cheng X., Zhang X., Pflugrath J.W.. The structure of bacteriophage T7 lysozyme, a zinc amidase and an inhibitor of T7 RNA polymerase. Proc Natl Acad Sci U S A 91:1994;4034−4038. PMID: 8171031.ArticlePubMedPMC
- 34. Yoon S.H., Kim S.K., Kim J.F.. Secretory production of recombinant proteins in Escherichia coli. Recent Pat Biotechnol 4:2010;23−29. PMID: 20201800.ArticlePubMed
- 35. Berkmen M.. Production of disulfide-bonded proteins in Escherichia coli. Protein Expres Purif 82:2012;240−251.Article
- 36. Schmelcher M., Shabarova T., Eugster M.R.. Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl Environ Microbiol 76:2010;5745−5756. PMID: 20622130.ArticlePubMedPMC
- 37. Fischetti V.A.. Bacteriophage lysins as effective anti-bacterials. Curr Opin Microbiol 11:2008;393−400. PMID: 18824123.ArticlePubMedPMC
- 38. Garcia P., Garcia J.L., Garcia E.. Modular organization of the lytic enzymes of Streptococcus pneumonia and its bacteriophages. Gene 86:1990;81−88. PMID: 2311937.ArticlePubMed
References
Figure 2Analysis of LysK-CHAP protein expression with two methods. (A) LysK-CHAP protein expression was analyzed with SDS-PAGE. (B) LysK-CHAP protein expression was analyzed with Western blot. C = control; CHAP = cysteine/histidine-dependent amidohydrolase/peptidase; SDS-PAGE = sodium dodecyl sulphate-polyacrylamide gel electrophoresis; M = protein Marker; S = protein sample.


Figure & Data
References
Citations
Citations to this article as recorded by 

- Production of mannooligosaccharides from orange peel waste with β-mannanase expressed in Trichosporonoides oedocephalis
Taotao Zhou, Xin Ju, Lishi Yan, Ruiqi Fang, Xinqi Xu, Liangzhi Li
Bioresource Technology.2024; 395: 130373. CrossRef - Chemotherapeutic Strategies for Combating Staphylococcus aureus Infections
Namita Sharma, Anil Kumar Chhillar, Sweety Dahiya, Aruna Punia, Pooja Choudhary, Prity Gulia, Akanksha Behl, Mehak Dangi
Mini-Reviews in Medicinal Chemistry.2022; 22(1): 26. CrossRef - Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases
H. Madanchi, M. Shoushtari, H.H. Kashani, S. Sardari
New Microbes and New Infections.2020; 34: 100627. CrossRef - Assessment of Relationship Between Expression of Survivin Protein and Histopathology Diagnosis and Malignancy Severity in Colon Specimen
Amin Jourabchin, Tahereh Mazoochi, Hamed Haddad Kashani, Tahereh Khamechian
Journal of Gastrointestinal Cancer.2020; 51(1): 76. CrossRef - The effect of calcium on the adhesion of Streptococcus mutans to Human Gingival Epithelial Cells in the presence of probiotic bacteria Lactobacillus plantarum and Lactobacillus salivarius
Mahboobeh Mehrabani Natanzi, Fatemeh Soleimanifard, Hamed Haddad Kashani, Mohammad Javad Azadchehr, Ahmadreza Mirzaei, Zohre Khodaii
Gene Reports.2020; 20: 100710. CrossRef - Expression and characterization of family 40 Carbohydrate Binding Module (CBM) from Vibrio cholerae Non-O1 sialidase
Gogula Selvi Asang, Shadariah Mamat, Nadiawati Alias, Asmad Kari
Asia Pacific Journal of Molecular Biology and Biot.2020; : 26. CrossRef - Effect of intraoperative dexmedetomidine infusion during functional endoscopic sinus surgery: a prospective cohort study
Mohammad Reza Fazel, Zeynab Sadat Ahmadi, Hossein Akbari, Fahimeh Abam
Patient Safety in Surgery.2020;[Epub] CrossRef - Comparison of a novel herbal skin care ointment with regular ointments to treat skin around the abdominal stoma: A clinical trial study
Maryam Hajikari, Soheila Mojdeh, Mohsen Shariari
Polish Annals of Medicine.2019;[Epub] CrossRef - Evaluation of the serum sex hormones levels and alkaline phosphatase activity in rats’ testis after administering of berberine in experimental varicocele
Hamed Najaran, Hassan Hassani Bafrani, Hamid Rashtbari, Fatemeh Izadpanah, Mohammad Reza Rajabi, Hamed Haddad Kashani, Abouzar Mohammadi
Oriental Pharmacy and Experimental Medicine.2019; 19(2): 157. CrossRef - Effect of melatonin in reducing second-generation antipsychotic metabolic effects: A double blind controlled clinical trial
Mansour Agahi, Negar Akasheh, Afshin Ahmadvand, Hossein Akbari, Fatemeh Izadpanah
Diabetes & Metabolic Syndrome: Clinical Research &.2018; 12(1): 9. CrossRef - Dengue viruses and promising envelope protein domain III-based vaccines
Hossein Fahimi, Mahshid Mohammadipour, Hamed Haddad Kashani, Farshid Parvini, Majid Sadeghizadeh
Applied Microbiology and Biotechnology.2018; 102(7): 2977. CrossRef - Recombinant Endolysins as Potential Therapeutics against Antibiotic-Resistant Staphylococcus aureus: Current Status of Research and Novel Delivery Strategies
Hamed Haddad Kashani, Mathias Schmelcher, Hamed Sabzalipoor, Elahe Seyed Hosseini, Rezvan Moniri
Clinical Microbiology Reviews.2018;[Epub] CrossRef - A Novel Chimeric Endolysin with Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus
Hamed Haddad Kashani, Hossein Fahimi, Yasaman Dasteh Goli, Rezvan Moniri
Frontiers in Cellular and Infection Microbiology.2017;[Epub] CrossRef - The lowering of bilirubin levels in patients with neonatal jaundice using massage therapy: A randomized, double-blind clinical trial
Fatemeh Eghbalian, Haneyeh Rafienezhad, Javad Farmal
Infant Behavior and Development.2017; 49: 31. CrossRef - The effect of chronic noise stress on serum levels of cortisol, gonadotropins, and sexual hormones at implantation time of mice
Alireza Shafiei, Hassan Ehteram, Hossein Akbari, Masoud Motalebi Kashani, Mandana Beigi, Javad Amini Mahabadi, Tahere Mazoochi
Comparative Clinical Pathology.2017; 26(4): 779. CrossRef - The Role of Probiotics in the Treatment of Dysentery: a Randomized Double-Blind Clinical Trial
Alireza Sharif, Hamed Haddad Kashani, Elahe Nasri, Zahra Soleimani, Mohammad Reza Sharif
Probiotics and Antimicrobial Proteins.2017; 9(4): 380. CrossRef - Assessment of 25-hydroxy vitamin D in stroke patients based on severity and type: a cross-sectional study
Ebrahim Kouchaki, Mansour Sayyah, Maryam Omidvari
Comparative Clinical Pathology.2017; 26(4): 811. CrossRef - Purification of Antibacterial CHAPK Protein Using a Self-Cleaving Fusion Tag and Its Activity Against Methicillin-Resistant Staphylococcus aureus
Elahe Seyed Hosseini, Rezvan Moniri, Yasaman Dasteh Goli, Hamed Haddad Kashani
Probiotics and Antimicrobial Proteins.2016; 8(4): 202. CrossRef


 PubReader
PubReader Cite
Cite
