Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 8(1); 2017 > Article
-
Original Article
A Novel PCR Assay for DetectingBrucella abortus andBrucella melitensis - Saeed Alamiana, Majid Esmaelizadb, Taghi Zahraeic, Afshar Etemadia, Mohsen Mohammadid, Davoud Afshare, Soheila Ghaderib
-
Osong Public Health and Research Perspectives 2017;8(1):65-70.
DOI: https://doi.org/10.24171/j.phrp.2017.8.1.09
Published online: February 28, 2017
aDepartment of Brucellosis, Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization, Karaj, Iran
bDepartment of Biotechnology, Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization, Karaj, Iran
cDepartment of Microbiology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
dDepartment of Pharmaceutical Biotechnology, Faculty of Pharmacy, Lorestan University of Medical Sciences, Khorramabad, Iran
eDepartment of Microbiology, Faculty of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
- Corresponding author: Saeed Alamian, E-mail: s.alamian@rvsri.ac.ir
Copyright © 2017 Korea Centers for Disease Control and Prevention
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- Brucellosis is a major zoonotic disease that poses a significant public health threat worldwide. The classical bacteriological detection process used to identify Brucella spp. is difficult and time-consuming. This study aimed to develop a novel molecular assay for detecting brucellosis.
-
Methods
- All complete sequences of chromosome 1 with 2.1-Mbp lengths were compared among all available Brucella sequences. A unique repeat sequence (URS) locus on chromosome 1 could differentiate Brucella abortus from Brucella melitensis. A primer set was designed to flank the unique locus. A total of 136 lymph nodes and blood samples were evaluated and classified by the URS-polymerase chain reaction (PCR) method in 2013–2014.
-
Results
- Biochemical tests and bacteriophage typing as the golden standard indicated that all Brucella spp. isolates were B. melitensis biovar 1 and B. abortus biovar 3. The PCR results were the same as the bacteriological method for detecting Brucella spp. The sensitivity and specificity of the URS-PCR method make it suitable for detecting B. abortus and B. melitensis.
-
Conclusion
- Quick detection of B. abortus and B. melitensis can provide the most effective strategies for control of these bacteria. The advantage of this method over other presented methods is that both B. abortus and B. melitensis are detectable in a single test tube. Furthermore, this method covered 100% of all B. melitensis and B. abortus biotypes. The development of this URS-PCR method is the first step toward the development of a novel kit for the molecular identification of B. abortus and B. melitensis.
- Brucella is a genus of aerobic nonmotile gram-negative coccobacilli [1]. Brucella melitensis and Brucella abortus are causative agents of brucellosis in small ruminant animals and cattle, respectively [2]. The most significant clinical signs of brucellosis in animals are abortion, reproductive disorders, and placental retention in females and orchitis and epididymitis in males [3]. Outbreaks of bovine brucellosis generally occur with abortion in the last 3 months of pregnancy and result in weak calves and bovine infertility [4]. Four species including Brucella canis, Brucella suis, B. abortus, and B. melitensis are human pathogens [5]. Human brucellosis is primarily a consequence of the contact with infected animals or consumption of unpasteurized dairy products. People in the Mediterranean, Middle East, and Latin American areas are at high risk of brucellosis [6–8]. The bacterium causes febrile septicemia or localized infection in the bone, tissues, and other organs in humans. Various reports from the endemic regions of B. melitensis showed an increased abortion incidence in pregnant women without any clinical signs [9].
- Diagnosis of Brucella strains in suspected samples is usually based on culture and serology tests. Identification of Brucella isolates at the species and biovar levels using classical bacterial methods is the gold standard, but is time-consuming and requires long incubation times and multiple phenotypical tests [10]. Different genes are candidates for the detection of brucellosis and identification of Brucella species using polymerase chain reaction (PCR) [11–15].
- Molecular methods for brucellosis detection are faster and more sensitive than traditional methods, but the sensitivity and specificity of PCR tests may be vary among laboratories [16].
- Many previously reported molecular methods for the isolation and differentiation of Brucella species are invalid and not applicable because of the deposit of many new genomic sequences of Brucella isolates since 2009. Thus, developing new molecular methods to differentiate species is crucial.
- The prevalence of brucellosis in Iran is caused by traditional husbandry of ruminant animals and poor sanitary equipment in rural areas. In humans and animals, the molecular detection of brucellosis is critical to meet epidemiological and preventive objectives. This study aimed to evaluate a novel PCR method for the identification and differentiation of B. melitensis and B. abortus. Due to some disadvantages of traditional brucellosis detection assays, development of new molecular methods that are more useful for detection, epidemiological, and surveillance studies is needed. Thus, this study focused on developing a novel PCR-based method for the identification and discrimination of two prevalent species of Brucella.
INTRODUCTION
- 1. Bacterial samples and growth conditions
- The present work was a molecular experimental study. The bacterial field strains used in this study are shown in Table 1. These strains include reference strains of Brucella species and bacterial strains that are serologically related to Brucella spp. In this study, 136 blood and lymph node samples were divided into two groups. Group 1 included a total of 48 human blood samples received from the Ministry of Health and Medical Education, while Group 2 included 88 bovine blood and lymph node samples from the Iranian Veterinary Organization during 2013–2014. Ethical approval was granted by the Razi Institute Agreement Committee in 2000 (no. Razi-1388). These samples were evaluated by bacteriological and PCR methods (Table 2).
- Clinical samples were received from different provinces of Iran. First, 10 mL of each human blood sample was cultured on Castaneda medium and incubated at 37°C for 21 days. Then, grown colonies were transferred to Brucella-specific agar and incubated at 37°C for 5–7 days. Bovine samples were cultured directly on Brucella-specific agar and incubated at 37°C for 21 days [17].
- 2. Brucella species phage typing
- Phage typing was done according to the method recommended by the World Health Organization. To initiate the growth, 10% CO2 was supplied; the H2S production was evaluated with lead acetate indicator. Acriflavin and crystal violet tests were used to discriminate between the smooth and rough Brucella strain colonies. Standard strains contain B. abortus biotype 1 (strain 544) and B. melitensis biotype 1 (strain 16 M) were used as control cultures. A dye sensitivity assay was performed in recommended solution as follows: thionin: 1/25,000, 1/50,000, 1/100,000; and basic fuchsin: 1/50,000, 1/100,000. We used Tb phages in the routine test dilution (RTD) and RTD × 10. Brucella cell wall antigens (A and M) were evaluated using monospecific anti-A and anti-M sera agglutination tests [17].
- 3. DNA extraction
- Bacterial cultured plates were washed with 5 mL phosphate buffered saline, and 100 μL of the bacterial suspension was centrifuged at 8,000 RPM at 4°C for 5 minutes and the supernatant was discarded. The DNA of all strains was extracted using Roche kit (Roche Applied Science, Mannheim, Germany) following the manufacturer’s instructions. The concentration of extracted DNA was determined by an ND-1000 spectrophotometer (Nano Drop, Wilmington, DE, USA).
- 4. Comparative genome analysis and primer design
- Nucleotide sequences of chromosome 1 with 2.1 Mbp lengths were compared among all of the Brucella species whole genome sequences from GenBank by online software such as Basic Local Alignment Search Tool. Primers were designed by in flanking of polymorphic locus with 100% coverage for all B. abortus and B. melitensis bacteria.
- 5. Unique repeat sequence (URS)-PCR assay
- In this study, UF1 and UR1 primers were used to detect and discriminate between B. melitensis and B. abortus. The PCR mixture used to detect B. abortus and B. melitensis included 10 pmol UF1 and UR1 primers, 50 ng DNA, 2.5 mM MgCl2, 1.25 units Taq DNA polymerase, 200 μM dNTP, 2.5 μL 10× PCR buffer, and up to 25 μL distilled water. The thermal cycle program was designed with one initial denaturation at 95°C for 4 minutes followed by 30 cycles of 94°C for 30 seconds, 52°C for 30 seconds, and 72°C for 45 seconds. The final extension step was performed at 72°C for 5 minutes. PCR products were visualized in 10% acrylamide gel electrophoresis and stained with silver nitrate.
- 6. Sensitivity assay
- Well-purified genomic DNA of reference strains, B. abortus 544 and B. melitensis 16 M, were prepared and 10-fold serial dilutions were made as follows: 500 ng/μL, 50 ng/μL, 5 ng/μL, 0.5 ng/μL, and 0.05 ng/μL. One microliter of each dilution was used as a template in the PCR assays.
- 7. Specificity assay
- To determine PCR specificity, a group of well-characterized Brucella and non-Brucella strains were evaluated (Table 1).
- 8. Statistical analysis
- All statistical analyses were performed by Gen ALEX 6.41 software (Table 3).
MATERIALS AND METHODS
- 1. Comparative genome analysis and URS-PCR
- Only one novel locus was found in chromosome 1 at nucleotide positions 1048645–1048562 of B. melitensis 16M. A comparative sequence study showed two repeat sequences (TCT TTG GGG GT) in all B. abortus strains, while only one repeat was observed in all of the B. melitensis strains. This locus had the capacity to design appropriate primers to differentiate between B. melitensis and B. abortus based on the full genome sequences of Brucella deposited before 2014 in GenBank. This locus is a URS included 15 nucleotide variations between B. abortus and B. melitensis. In this study, a primer set comprising forward UF1 (5′-GGC TAT CGG CTG GGA AAG G-3′) and reverse UR1 (5′-CCT TCC GAA GAA AAT ACC CCT-3′) was designed to flank the polymorphic repeat sequence region. Two specific amplicons (84 bp and 99 bp long) were produced for the detection of B. melitensis and B. abortus, respectively. These primers covered all intraspecies biovars based on available sequences in nucleotide databases.
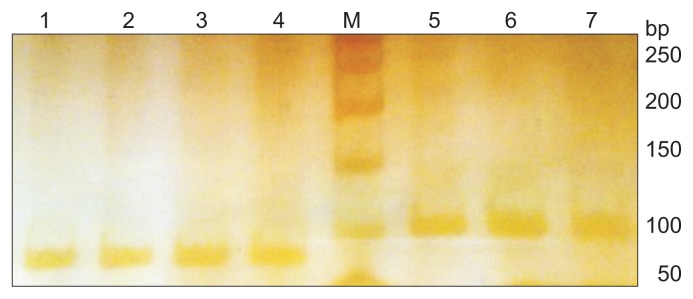
- A total of 136 Brucella (48 human and 88 bovine) isolates were evaluated (Tables 2 and 3). The Brucella strains were typed by biochemical and standard phage typing methods using the Tb phage as described by Alton et al [17]. The bacteriological typing results indicated that all the human isolates were categorized into B. melitensis biovar 1 and B. abortus biovar 3 in samples received from 2014 (Tables 2 and 3). Genomic DNA of B. melitensis and B. abortus were amplified by the UF1 and UR1 primers. The amplicons were 84 bp and 99 bp long for B. melitensis and B. abortus, respectively, and visualized in 10% acrylamide gel electrophoresis using the silver staining method (Figure 1).
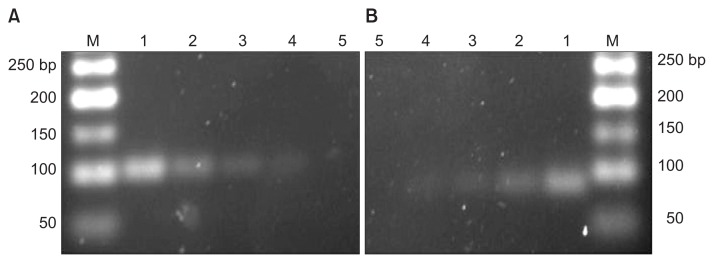
- This PCR assay detected all B. abortus reference and field strains that were classified using the bacteriological method as the gold standard; thus, the sensitivity was 100% for B. abortus 544 and the limit of detection was 0.5 ng of genomic DNA. B. melitensis (reference and field strains) were detected by the URS-PCR method, whose results were in agreement with those of the conventional bacteriological method. Furthermore, the limit of detection was 0.65 ng of genomic DNA (Figure 2).
RESULTS
- Acute febrile illness (AFI) is an important clinical syndrome that requires supportive treatment. Brucella, the causative pathogens of AFI, is considered a critical issue in public health in developing countries such as Iran. Most people are threatened by brucellosis because of their traditional lifestyles [18]. The incidence of human brucellosis is directly related to the prevalence of animal brucellosis in specific regions, while the actual rate of human brucellosis is estimated to be 10–25 times higher than those reported [19–21]. Brucellosis remains endemic in most Mediterranean and Middle East countries despite all preventative procedures in recent decades. The detection of native Brucella species biovars in infected animals and humans is critical for establishing preventive factors and controlling the disease.
- The first isolate of Brucella was identified in a bovine fetus (B. abortus biovar 3) in Iran in 1944 [22–24]. In an epidemiological study, 3,031 Brucella isolates were characterized by the standard phage typing method. All Iranian B. abortus isolates were grouped into seven biovars (1–6 and 9). A dominant strain was B. abortus biovar 3 in Iran. In Turkey, B. abortus biovar 3 is dominant in dairy farms as well [25].
- According to epidemiological studies, species and isolates are similar in this region. However, the first B. melitensis isolate (biovar 1) was identified in Iran 6 years after the first B. abortus isolation in 1950 [26,27]. B. melitensis biovars 1–3 were identified in Iran, but biovar 1 was dominant in human brucellosis. Previous studies by Zowghi et al [28] and Khosravi et al [29] showed that B. melitensis biovar 1 was dominant in human brucellosis in Iran. The gold standard for the diagnosis of brucellosis in humans and animals is based on the isolation of Brucella bacteria [30]. Due to some limitations in the isolation of Brucella bacteria, such as the need for high biosafety level facilities, personnel skill, and risk of laboratory infection, several molecular methods to improve sensitivity and specificity, decrease cost, offer the rapid brucellosis detection, identify and differentiate Brucella species have been developed [31].
- In previous studies, different primers were designed for the detection of all intraspecies biovars of B. melitensis and B. abortus [11–15], but they had insufficient efficacy against all intraspecies biovars based on new deposited whole genome sequences. In this study, two novel primers were designed to flank a unique locus on chromosome 1, while a single URS-PCR was developed to simultaneously identify and differentiate between B. abortus and B. melitensis at the species level. The URS-PCR results showed 100% agreement with those of the conventional phage typing method. This technique covered all biovars.
- Brucellosis is worldwide zoonotic disease that causes several economic and public health problems. Control and eradication of this disease is dependent upon its rapid detection and monitoring. As such, access to a fast and accurate method of identifying the causative agent is important. Note that bacteriological methods are time-consuming and require special equipment and conditions for the detection of Brucella strains. Because of the high similarity among species within the Brucella genus, discrimination is problematic.
- Based on the results of an in silico study on Brucella chromosomes, we found repeat sequences that can be used for Brucella intraspecies detection. Thus, we suggest that this novel URS-PCR method that was designed based on a URS in chromosome 1 be used for the rapid detection of B. abortus and B. melitensis. The advantage of this method over other presented methods is that both B. abortus and B. melitensis are detectable in a single test tube. Unlike methods in previous studies, this method covered 100% of all B. melitensis and B. abortus biotypes.
DISCUSSION
-
Acknowledgements
- This project was supported by grant no. 2-18-18-91124 of the Razi Vaccine and Serum Research Institute. The authors wish to thank the personnel working in the Brucellosis and Biotechnology departments. Also we are particularly grateful to Dr. Khosro Aghaeipour for their valuable comments and sharing their knowledge.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
- 1. Young EJ. An overview of human brucellosis. Clin Infect Dis 1995;283−9. quiz 290. https://doi.org/10.1093/clinids/21.2.283. PMID: 10.1093/clinids/21.2.283. PMID: 8562733.ArticlePubMed
- 2. Al-Ani FK, El-Qaderi S, Hailat NQ, et al. Human and animal brucellosis in Jordan between 1996 and 1998: a study. Rev Sci Tech 2004;23:831−40. PMID: 15861878.ArticlePubMed
- 3. Refai M. Incidence and control of brucellosis in the Near East region. Vet microbiol 2002;90:81−110. https://doi.org/10.1016/S0378-1135(02)00248-1. PMID: 10.1016/S0378-1135(02)00248-1. PMID: 12414137.ArticlePubMed
- 4. Fekete A, Bantle JA, Halling SM. Detection of Brucella by polymerase chain reaction in bovine fetal and maternal tissues. J Vet Diagn Invest 1992;4:79−83. PMID: 10.1177/104063879200400118. PMID: 1554774.ArticlePubMed
- 5. Kamal IH, Al Gashgari B, Moselhy SS, et al. Two-stage PCR assay for detection of human brucellosis in endemic areas. BMC Infect Dis 2013;13:145https://doi.org/10.1186/1471-2334-13-145. PMID: 10.1186/1471-2334-13-145. PMID: 23517532.ArticlePubMedPMC
- 6. Arnow PM, Smaron M, Ormiste V. Brucellosis in a group of travelers to Spain. JAMA 1984;251:505−7. https://doi.org/10.1001/jama.1984.03340280055029. PMID: 10.1001/jama.1984.03340280055029. PMID: 6690819.ArticlePubMed
- 7. Gedikoğlu S, Helvaci S, Ozakin C, et al. Detection of Brucella melitensis by BACTEC NR 730 and BACTEC 9120 systems. Eur J Epidemiol 1996;12:649−50. https://doi.org/10.1007/BF00499467. PMID: 10.1007/BF00499467. PMID: 8982628.ArticlePubMed
- 8. Yagupsky P. Detection of brucellae in blood cultures. J Clin Microbiol 1999;37:3437−42. PMID: 10523530.ArticlePubMedPMC
- 9. Boschiroli ML, Foulongne V, O’Callaghan D. Brucellosis: a worldwide zoonosis. Curr Opin Microbiol 2001;4:58−64. https://doi.org/10.1016/S1369-5274(00)00165-X. PMID: 10.1016/S1369-5274(00)00165-X. PMID: 11173035.ArticlePubMed
- 10. Poester FP, Nielsen K, Samartino LE, et al. Diagnosis of brucellosis. Open Vet Sci J 2010;4:46−60. PMID: 10.2174/1874318801004010046.Article
- 11. Baily GG, Krahn JB, Drasar BS, et al. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J Trop Med Hyg 1992;95:271−5. PMID: 1495123.PubMed
- 12. Fekete A, Bantle JA, Halling SM, et al. Amplification fragment length polymorphism in Brucella strains by use of polymerase chain reaction with arbitrary primers. J Bacteriol 1992;174:7778−83. https://doi.org/10.1128/jb.174.23.7778-7783.1992. PMID: 10.1128/jb.174.23.7778-7783.1992. PMID: 1360006.ArticlePubMedPMC
- 13. Herman L, De Ridder H. Identification of Brucella spp. by using the polymerase chain reaction. Appl Environ Microbiol 1992;58:2099−101. PMID: 1377903.ArticlePubMedPMC
- 14. Romero C, Gamazo C, Pardo M, et al. Specific detection of Brucella DNA by PCR. J Clin Microbiol 1995;33:615−7. PMID: 7538508.ArticlePubMedPMC
- 15. Leal-Klevezas DS, Martínez-Vázquez IO, López-Merino A, et al. Single-step PCR for detection of Brucella spp. from blood and milk of infected animals. J Clin Microbiol 1995;33:3087−90. PMID: 8586678.ArticlePubMedPMC
- 16. Navarro E, Casao MA, Solera J. Diagnosis of human brucellosis using PCR. Exp Rev Mol Diagn 2004;4:115−23. https://doi.org/10.1586/14737159.4.1.115. PMID: 10.1586/14737159.4.1.115.Article
- 17. Alton G, Jones L, Pietz D. Laboratory techniques in brucellosis. Monogr Ser World Health Organ 1975;(55). 1−163. PMID: 812265.PubMed
- 18. Minas M, Minas A, Gourgulianis K, et al. Epidemiological and clinical aspects of human brucellosis in Central Greece. Jpn J Infect Dis 2007;60:362−6. PMID: 18032835.ArticlePubMed
- 19. Memish ZA, Almuneef M, Mah MW, et al. Comparison of the Brucella Standard Agglutination Test with the ELISA IgG and IgM in patients with Brucella bacteremia. Diagn Microbiol Infect Dis 2002;44:129−32. https://doi.org/10.1016/S0732-8893(02)00426-1. PMID: 10.1016/S0732-8893(02)00426-1. PMID: 12458117.ArticlePubMed
- 20. Godfroid J, Cloeckaert A, Liautard JP, et al. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res 2005;36:313−26. https://doi.org/10.1051/vetres:2005003. PMID: 10.1051/vetres:2005003. PMID: 15845228.ArticlePubMed
- 21. Corbel MJ. Brucellosis: an overview. Emerg Infect Dis 1997;3:213−21. https://doi.org/10.3201/eid0302.970219. PMID: 10.3201/eid0302.970219. PMID: 9204307.ArticlePubMedPMC
- 22. Delpy L, Kaveh M. The occurrence of brucellosis in Iran. The isolation of the causative agent of contagious abortion in cattle. Rev Fac Med Vet Teheran 1945;1:21−9.
- 23. Zowghi E, Ebadi A, Vandyousefi D. Bacteriological investigations of bovine, ovine and caprine brucellosis in Iran. Rev Sci Tech Off Int Epiz 1984;3:583−8. PMID: 10.20506/rst.3.3.171.Article
- 24. Zowghi E, Ebadi A. Abortion due to brucella abortus in sheep in Iran. Rev Sci Tech 1988;7:379−82.ArticlePubMed
- 25. Buyukcangaz E, Sen A. The first isolation of Brucella melitensis from bovine aborted fetus in Turkey. J Biol Environ Sci 2007;1:139−42.
- 26. Kaveh M. Brucellosis in general. Rev Fac Med Vet Téheran 1952;1:15−66.
- 27. Etemady A, Mohammdi M, Esmaelizad M, et al. Genetic characterization of the wboA gene from the predominant biovars of Brucella isolates in Iran. Electron Physician 2015;7:1381−6. https://doi.org/10.14661/1381. PMID: 26516446.ArticlePubMedPMC
- 28. Zowghi E, Ebadi A, Yarahmadi M. Isolation and identification of Brucella organisms in Iran. Arch Clin Infect Dis 2009;3:185−8.
- 29. Khosravi AD, Abasi E, Alavi SM. Isolation of Brucella melitensis and Brucella abortus from brucellosis patients by conventional culture method and polymerase chain reaction technique. Pak J Med Sci 2006;22:396−400.
- 30. Yagupsky P. Detection of Brucella melitensis by BACTEC NR660 blood culture system. J Clin Microbiol 1994;32:1899−901. PMID: 7989539.ArticlePubMedPMC
- 31. Nagalingam M, Shome R, Balamurugan V, et al. Molecular typing of Brucella species isolates from livestock and human. Trop Anim Health Prod 2012;44:5−9. https://doi.org/10.1007/s11250-011-9886-1. PMID: 10.1007/s11250-011-9886-1. PMID: 21647774.ArticlePubMed
REFERENCES
| Year | Samples | No. of Brucella-positive samples | Species frequencies | |
|---|---|---|---|---|
| B. abortus 3 | B. melitensis 1 | |||
| 2013 | Bovine | 45 | 0.978 | 0.022 |
| Human | 27 | 0.00 | 1.00 | |
| 2014 | Bovine | 43 | 1.00 | 0.00 |
| Human | 21 | 0.00 | 1.00 | |
Figure & Data
References
Citations

- Development of a simplified and cost-effective sample preparation method for genotyping of human papillomavirus by next-generation sequencing
Rungrat Jitvaropas, Ukrit Thongpoom, Vorthon Sawaswong, Kritsada Khongnomnan, Witthaya Poomipak, Kesmanee Praianantathavorn, Pornjarim Nilyanimit, Yong Poovorawan, Sunchai Payungporn
Archives of Virology.2023;[Epub] CrossRef - Bovine brucellosis – a comprehensive review
Sandip Kumar Khurana, Anju Sehrawat, Ruchi Tiwari, Minakshi Prasad, Baldev Gulati, Muhammad Zubair Shabbir, Rajesh Chhabra, Kumaragurubaran Karthik, Shailesh Kumar Patel, Mamta Pathak, Mohd. Iqbal Yatoo, Vivek Kumar Gupta, Kuldeep Dhama, Ranjit Sah, Wanpe
Veterinary Quarterly.2021; 41(1): 61. CrossRef - Survey of Zoonotic Bacterial Pathogens in Native Foxes in Central Chile: First Record of Brucella canis Exposure
Nicolás Galarce, Sebastián de la Fuente, Beatriz Escobar, Phillip Dettleff, Pedro Abalos, Juan Carlos Hormazábal, Roberto Flores, Nicole Sallaberry-Pincheira, Víctor Martínez
Animals.2021; 11(7): 1980. CrossRef - Development and validation of immunoassay for whole cell detection of Brucella abortus and Brucella melitensis
Richa Hans, Pranjal Kumar Yadav, Pushpendra Kumar Sharma, Mannan Boopathi, Duraipandian Thavaselvam
Scientific Reports.2020;[Epub] CrossRef - Laboratory Diagnostic Procedures for Human Brucellosis: An Overview of Existing Approaches
Afshar Etemadi, Rezvan Moniri, Heinrich Neubauer, Yasaman Dasteh Goli, Saeed Alamian
Jundishapur Journal of Microbiology.2019;[Epub] CrossRef - Comparison of PCR-RFLP and PFGE for determining the clonality of Brucella isolates from human and livestock specimens
Nasrin Bahmani, Reza Mirnejad, Mohammad Reza Arabestani, Parviz Mohajerie, Seyed Hamid Hashemi, Manoochehr Karami, Mohammad Yousef Alikhani
Saudi Journal of Biological Sciences.2019; 26(2): 256. CrossRef -
Designing an immunosensor for detection of
Brucella abortus
based on coloured silica nanoparticles
Arash Shams, Bahareh Rahimian Zarif, Mojtaba Salouti, Reza Shapouri, Sako Mirzaii
Artificial Cells, Nanomedicine, and Biotechnology.2019; 47(1): 2562. CrossRef - Identification of Brucella genus and eight Brucella species by Luminex bead-based suspension array
Tina S. Lusk Pfefer, Ruth Timme, Julie A. Kase
Food Microbiology.2018; 70: 113. CrossRef


 PubReader
PubReader Cite
Cite
