Search
- Page Path
- HOME > Search
Original Articles
- Effect of Paxlovid in COVID-19 treatment during the periods of SARS-CoV-2 Omicron BA.5 and BN.1 subvariant dominance in the Republic of Korea: a retrospective cohort study
- Dong-Hwi Kim, Min-Gyu Yoo, Na-Young Kim, So Young Choi, Minjeong Jang, Misuk An, Se-Jin Jeong, Jungyeon Kim
- Received August 16, 2023 Accepted January 18, 2024 Published online March 28, 2024
- DOI: https://doi.org/10.24171/j.phrp.2023.0230 [Epub ahead of print]
- 273 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF - Objectives
This study was conducted to assess the efficacy of nirmatrelvir/ritonavir treatment in patients with coronavirus disease 2019 (COVID-19), particularly those aged 60 years and older. Using real-world data, the period during which the BN.1 Omicron variant was dominant was compared to the period dominated by the BA.5 variant.
Methods
In this retrospective cohort study, data were collected regarding 2,665,281 patients infected with severe acute respiratory syndrome coronavirus 2 between July 24, 2022, and March 31, 2023. Propensity score matching was utilized to match patients who received nirmatrelvir/ritonavir in a 1:4 ratio between BN.1 and BA.5 variant groups. Multivariable logistic regression analysis was employed to assess the effects of nirmatrelvir/ritonavir within these groups.
Results
Compared to the prior period, the efficacy of nirmatrelvir/ritonavir did not significantly differ during the interval of Omicron BN.1 variant dominance in the Republic of Korea. Among patients treated with nirmatrelvir/ritonavir, a significantly lower risk of mortality was observed in the BN.1 group (odds ratio [OR], 0.698; 95% confidence interval [CI], 0.557–0.875) compared to the BA.5 group. However, this treatment did not significantly reduce the risk of severe or critical illness, including death, for those in the BN.1 group (OR, 0.856; 95% CI, 0.728–1.007).
Conclusion
Nirmatrelvir/ritonavir has maintained its effectiveness against COVID-19, even with the emergence of the BN.1 Omicron subvariant. Consequently, we strongly recommend the administration of nirmatrelvir/ritonavir to patients exhibiting COVID-19-related symptoms, irrespective of the dominant Omicron variant or their vaccination status, to mitigate disease severity and decrease the risk of mortality.
- COVID-19 infection among people with disabilities in 2021 prior to the Omicron-dominant period in the Republic of Korea: a cross-sectional study
- Seul-Ki Kang, Bryan Inho Kim
- Received July 11, 2023 Accepted January 16, 2024 Published online March 28, 2024
- DOI: https://doi.org/10.24171/j.phrp.2023.0194 [Epub ahead of print]
- 407 View
- 8 Download
-
 Abstract
Abstract
 PDF
PDF - Objectives
This study investigated the characteristics of coronavirus disease 2019 (COVID-19) among individuals with disabilities on a nationwide scale in the Republic of Korea, as limited research has examined this population.
Methods
Between January 1 and November 30, 2021, a total of 5,687 confirmed COVID-19 cases among individuals with disabilities were reported through the Korea Disease Control and Prevention Agency’s COVID-19 web reporting system. Follow-up continued until December 24, and demographic, epidemiological, and clinical characteristics were analyzed.
Results
Individuals with disabilities represented approximately 1.5% of confirmed cases, with a mean age of 58.1 years. Most resided in or near metropolitan areas (86.6%) and were male (60.6%). Frequent sources of infection included home (33.4%) and contact with confirmed cases (40.7%). Many individuals (75.9%) had underlying conditions, and 7.7% of cases were severe. People with disabilities showed significantly elevated risk of severe infection (adjusted odds ratio [aOR], 1.63; 95% confidence interval [CI], 1.47–1.81) and mortality (aOR, 1.65; 95% CI, 1.43–1.91). Vaccination against COVID-19 was associated with significantly lower risk of severe infection (aORs for the first, second, and third doses: 0.6 [95% CI, 0.42–0.85], 0.28 [95% CI, 0.22–0.35], and 0.16 [95% CI, 0.05–0.51], respectively) and death (adjusted hazard ratios for the first and second doses: 0.57 [95% CI, 0.35–0.93] and 0.3 [95% CI, 0.23–0.40], respectively).
Conclusion
Individuals with disabilities showed higher risk of severe infection and mortality from COVID-19. Consequently, it is critical to strenghthenCOVID-19 vaccination initiatives and provide socioeconomic assistance for this vulnerable population.
- Risk factors for SARS-CoV-2 transmission during a movie theater outbreak in Incheon in the Republic of Korea, November 2021: a retrospective study
- Hye Young Lee, Young-Joon Park, Sang-Eun Lee, Han-Na Yoo, Il-Hwan Kim, Jin Sun No, Eun-Jin Kim, Jungyeon Yu, Sanghwan Bae, Mi Yu
- Osong Public Health Res Perspect. 2024;15(1):45-55. Published online January 31, 2024
- DOI: https://doi.org/10.24171/j.phrp.2023.0269
- 10,582 View
- 325 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 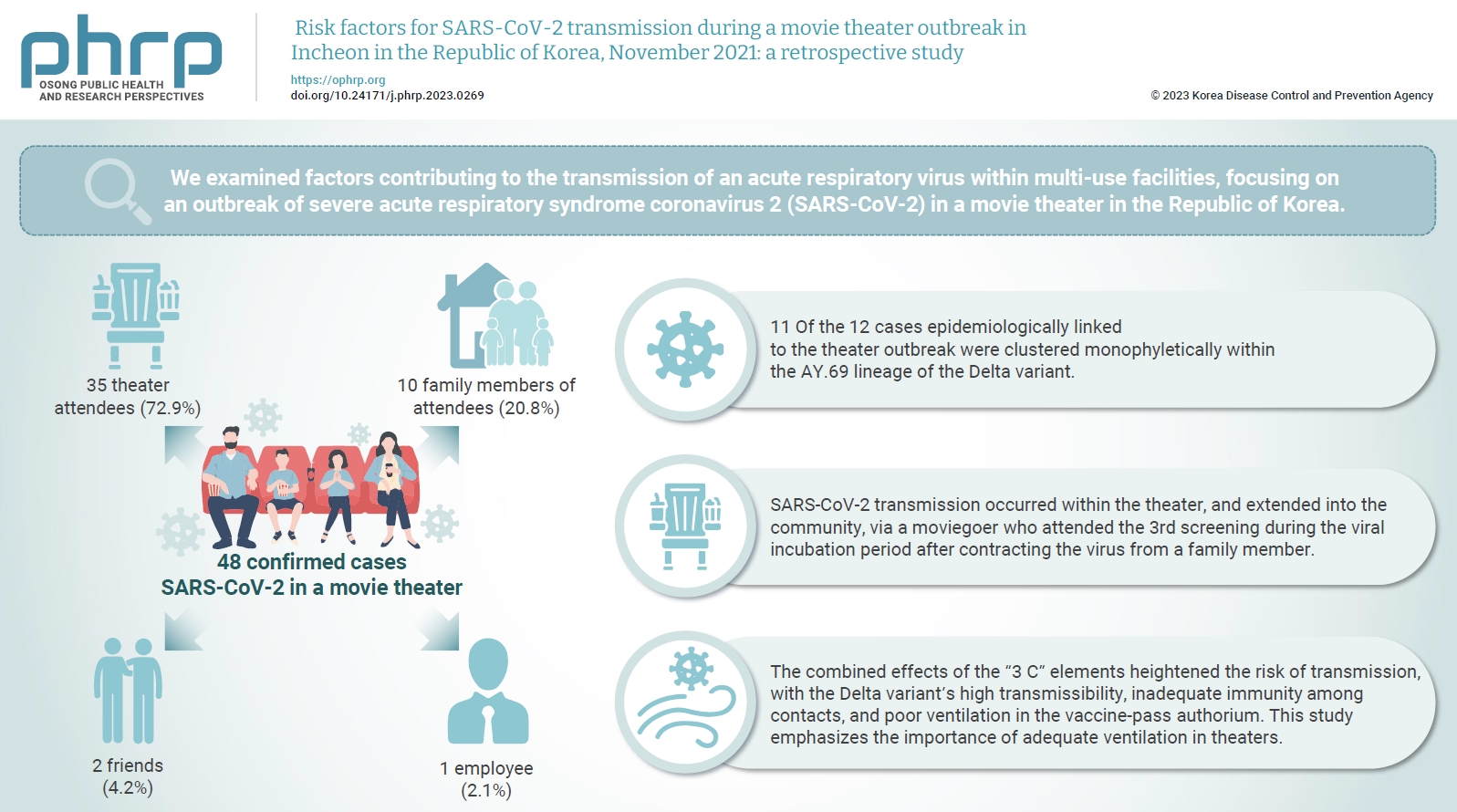
- Objectives
We examined factors contributing to the transmission of an acute respiratory virus within multi-use facilities, focusing on an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a movie theater in the Republic of Korea. Methods: This retrospective cohort study involved a descriptive analysis of 48 confirmed cases. Logistic regression was applied to a cohort of 80 theater attendees to identify risk factors for infection. The infection source and transmission route were determined through gene sequencing data analysis. Results: Of the 48 confirmed cases, 35 were theater attendees (72.9%), 10 were family members of attendees (20.8%), 2 were friends (4.2%), and 1 was an employee (2.1%). Among the 80 individuals who attended the 3rd to 5th screenings of the day, 35 became infected, representing a 43.8% attack rate. Specifically, 28 of the 33 third-screening attendees developed confirmed SARSCoV-2, constituting an 84.8% attack rate. Furthermore, 11 of the 12 cases epidemiologically linked to the theater outbreak were clustered monophyletically within the AY.69 lineage. At the time of the screening, 35 individuals (72.9%) had received 2 vaccine doses. However, vaccination status did not significantly influence infection risk. Multivariate analysis revealed that close contacts had a 15.9-fold higher risk of infection (95% confidence interval, 4.37–78.39) than casual contacts. Conclusion: SARS-CoV-2 transmission occurred within the theater, and extended into the community, via a moviegoer who attended the 3rd screening during the viral incubation period after contracting the virus from a family member. This study emphasizes the importance of adequate ventilation in theaters.
- Genetic diversity and evolutionary patterns of SARS-CoV-2 among the Bhutanese population during the pandemic
- Tshering Dorji, Kunzang Dorji, Tandin Wangchuk, Tshering Pelki, Sonam Gyeltshen
- Osong Public Health Res Perspect. 2023;14(6):494-507. Published online December 14, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0209
- 1,167 View
- 48 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 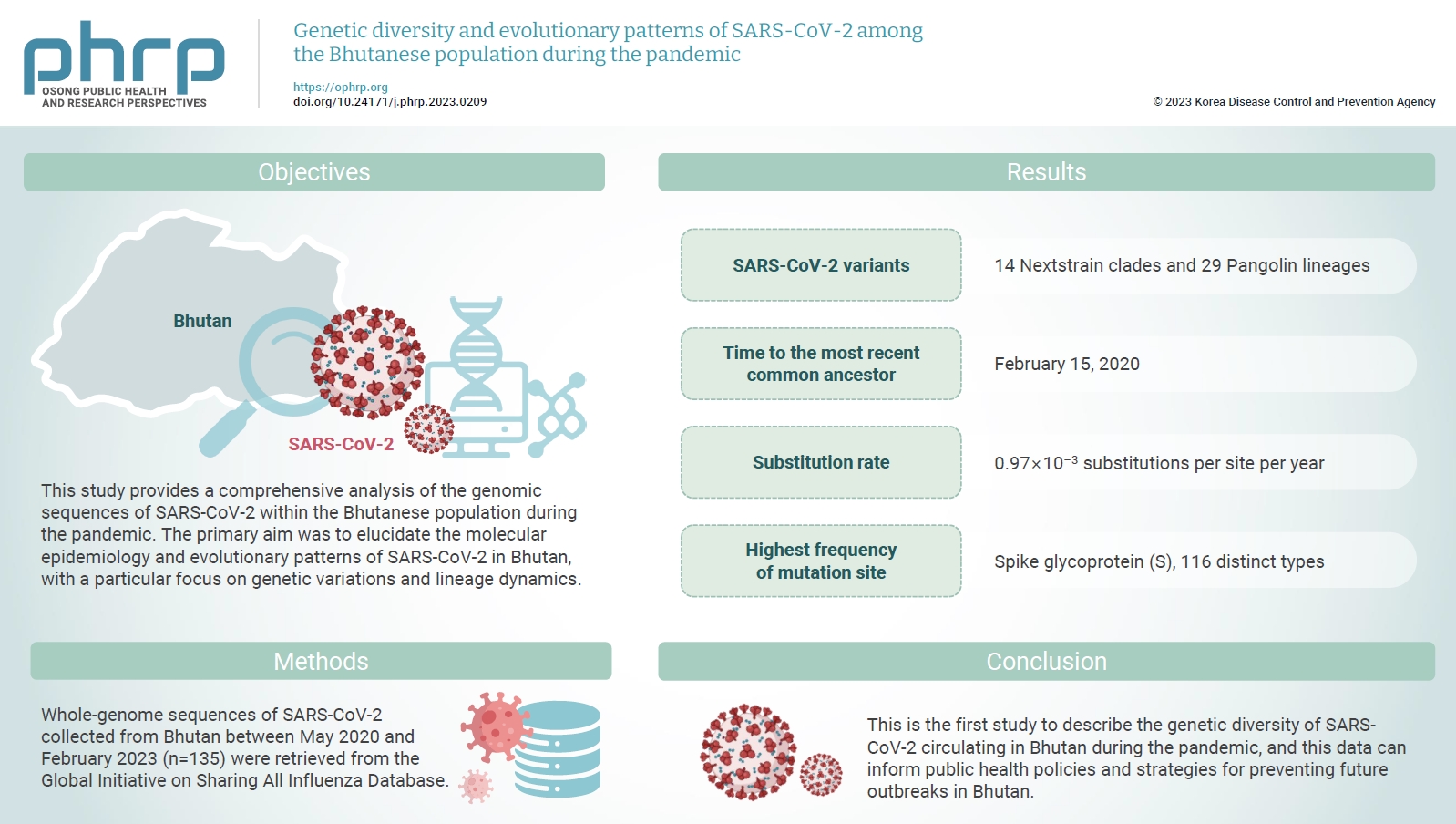
- Objectives
The coronavirus disease 2019 (COVID-19) pandemic, caused by a dynamic virus, has had a profound global impact. Despite declining global COVID-19 cases and mortality rates, the emergence of new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants remains a major concern. This study provides a comprehensive analysis of the genomic sequences of SARS-CoV-2 within the Bhutanese population during the pandemic. The primary aim was to elucidate the molecular epidemiology and evolutionary patterns of SARS-CoV-2 in Bhutan, with a particular focus on genetic variations and lineage dynamics. Methods: Whole-genome sequences of SARS-CoV-2 collected from Bhutan between May 2020 and February 2023 (n=135) were retrieved from the Global Initiative on Sharing All Influenza Database. Results: The SARS-CoV-2 variants in Bhutan were predominantly classified within the Nextstrain clade 20A (31.1%), followed by clade 21L (20%) and clade 22D (15.6%). We identified 26 Pangolin lineages with variations in their spatial and temporal distribution. Bayesian time-scaled phylogenetic analysis estimated the time to the most recent common ancestor as February 15, 2020, with a substitution rate of 0.97×10–3 substitutions per site per year. Notably, the spike glycoprotein displayed the highest mutation frequency among major viral proteins, with 116 distinct mutations, including D614G. The Bhutanese isolates also featured mutations such as E484K, K417N, and S477N in the spike protein, which have implications for altered viral properties. Conclusion: This is the first study to describe the genetic diversity of SARS-CoV-2 circulating in Bhutan during the pandemic, and this data can inform public health policies and strategies for preventing future outbreaks in Bhutan.
- Evaluation of COVID-19 vaccine effectiveness in different high-risk facility types during a period of Delta variant dominance in the Republic of Korea: a cross-sectional study
- Min Jei Lee, Myung-Jae Hwang, Dong Seob Kim, Seon Kyeong Park, Jihyun Choi, Ji Joo Lee, Jong Mu Kim, Young-Man Kim, Young-Joon Park, Jin Gwack, Sang-Eun Lee
- Osong Public Health Res Perspect. 2023;14(5):418-426. Published online October 19, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0188
- 1,278 View
- 44 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 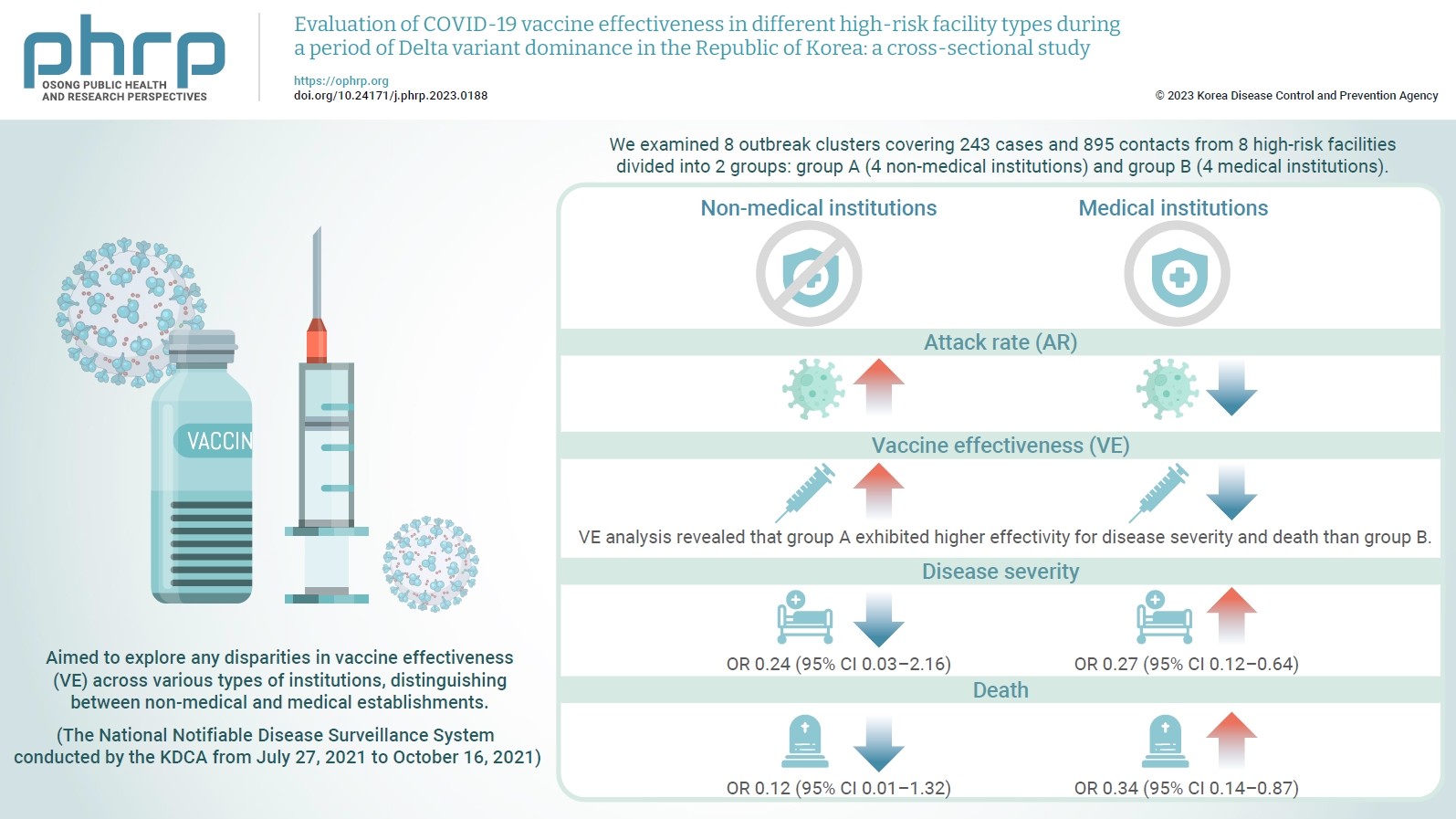
- Objectives
We evaluated the effectiveness of coronavirus disease 2019 vaccination in high-risk facilities in the Republic of Korea during the period when the highly transmissible Delta variant was prevalent. Additionally, we aimed to explore any disparities in vaccine effectiveness (VE) across various types of institutions, specifically distinguishing between non-medical and medical establishments. Methods: We examined 8 outbreak clusters covering 243 cases and 895 contacts from 8 high-risk facilities divided into 2 groups: group A (4 non-medical institutions) and group B (4 medical institutions). These clusters were observed from July 27, 2021 to October 16, 2021 for the attack rate (AR) and VE with respect to disease severity. A generalized linear model with a binomial distribution was used to determine the odds ratio (OR) for disease severity and death. Results: AR was notably lower in group B (medical institutions). Furthermore, VE analysis revealed that group A exhibited higher effectivity for disease severity and death than group B. The OR for disease severity was 0.24 (95% confidence interval [CI], 0.03–2.16) for group A and 0.27 (95% CI, 0.12–0.64) for group B, with the OR for death at 0.12 (95% CI, 0.01–1.32) in group A and 0.34 (95% CI, 0.14–0.87) in group B. Conclusion: Although VE may vary across institutions, our findings underscore the importance of implementing vaccinations in high-risk facilities. Customized vaccination programs, tailored response plans, and competent management personnel are essential for effectively addressing and mitigating public health challenges.
- Estimating the prevalence of oral manifestations in COVID-19 patients: a systematic review
- Ankita Gupta, Kriti Shrivastav, Amit Agrawal, Abhishek Purohit, Roshan Chanchlani
- Osong Public Health Res Perspect. 2023;14(5):388-417. Published online September 19, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0033
- 2,814 View
- 94 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 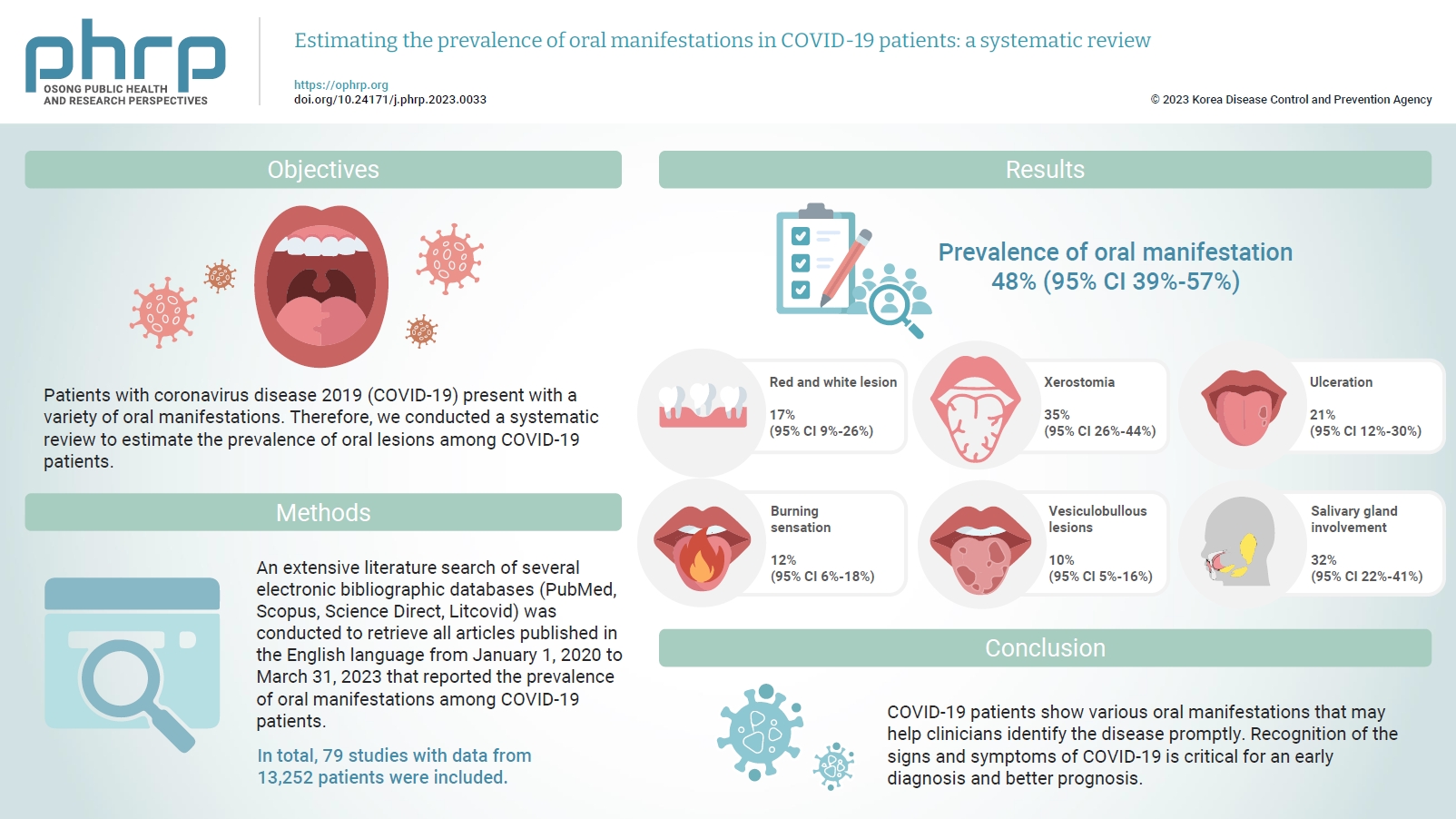
- Objectives
Patients with coronavirus disease 2019 (COVID-19) present with a variety of oral manifestations. Therefore, we conducted a systematic review to estimate the prevalence of oral lesions among COVID-19 patients. Methods: An extensive literature search of several electronic bibliographic databases (PubMed, Scopus, Science Direct, Litcovid) was conducted to retrieve all articles published in the English language from January 1, 2020 to March 31, 2023 that reported the prevalence of oral manifestations among COVID-19 patients. A meta-analysis of pooled prevalence was performed using Jamovi ver. 2.3 (2022). The I2 and Q statistics were used to assess heterogeneity between studies, and p-values <0.01 were considered statistically significant. Results: In total, 79 studies with data from 13,252 patients were included. The articles were predominantly published in 2020 (n=33), and Italy was the most common country (n=14). Most of the affected patients more than 50 years old and women (56.6%). The most common sites of involvement were the tongue (n=65), followed by the oral mucosa (n=37) and lips (n=19). High heterogeneity was found between studies. The most common oral manifestation was taste alteration, followed by xerostomia and ulceration, showing pooled prevalence rates of 48%, 35%, and 21%, respectively. Conclusion: COVID-19 patients show various oral manifestations that may help clinicians identify the disease promptly. Recognition of the signs and symptoms of COVID-19 is critical for an early diagnosis and better prognosis.
- Increased viral load in patients infected with severe acute respiratory syndrome coronavirus 2 Omicron variant in the Republic of Korea
- Jeong-Min Kim, Dongju Kim, Nam-Joo Lee, Sang Hee Woo, Jaehee Lee, Hyeokjin Lee, Ae Kyung Park, Jeong-Ah Kim, Chae Young Lee, Il-Hwan Kim, Cheon Kwon Yoo, Eun-Jin Kim
- Osong Public Health Res Perspect. 2023;14(4):272-278. Published online July 27, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0024
- 1,329 View
- 108 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 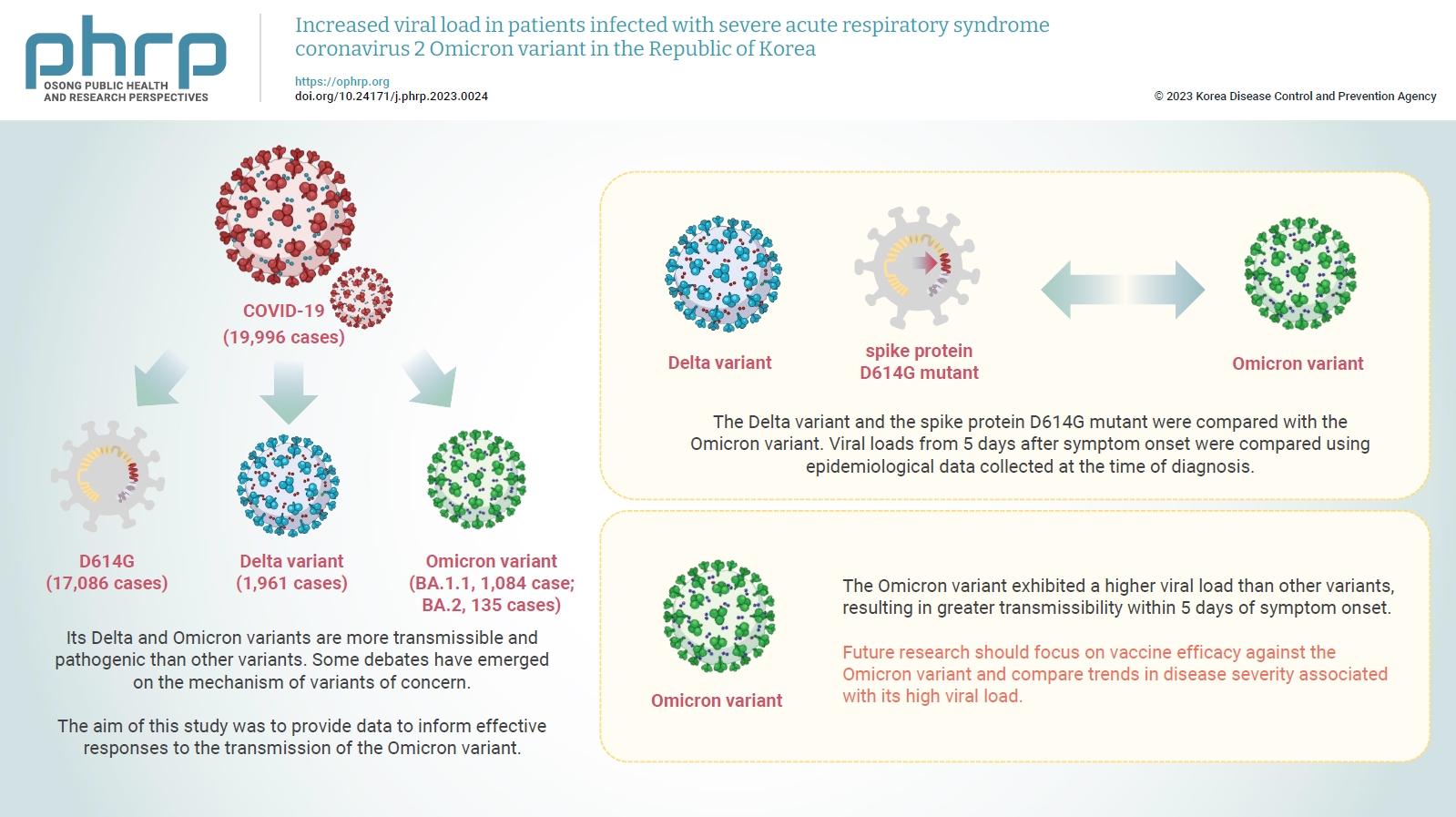
- Objectives
Coronavirus disease 2019 (COVID-19) has been declared a global pandemic owing to the rapid spread of the causative agent, severe acute respiratory syndrome coronavirus 2. Its Delta and Omicron variants are more transmissible and pathogenic than other variants. Some debates have emerged on the mechanism of variants of concern. In the COVID-19 wave that began in December 2021, the Omicron variant, first reported in South Africa, became identifiable in most cases globally. The aim of this study was to provide data to inform effective responses to the transmission of the Omicron variant.
Methods
The Delta variant and the spike protein D614G mutant were compared with the Omicron variant. Viral loads from 5 days after symptom onset were compared using epidemiological data collected at the time of diagnosis.
Results
The Omicron variant exhibited a higher viral load than other variants, resulting in greater transmissibility within 5 days of symptom onset.
Conclusion
Future research should focus on vaccine efficacy against the Omicron variant and compare trends in disease severity associated with its high viral load.
- Estimating the number of severe COVID-19 cases and COVID-19-related deaths averted by a nationwide vaccination campaign in Republic of Korea
- Ji Hae Hwang, Ju Hee Lee, Eun Jung Jang, Ryu Kyung Kim, Kil Hun Lee, Seon Kyeong Park, Sang Eun Lee, Chungman Chae, Sangwon Lee, Young Joon Park
- Osong Public Health Res Perspect. 2023;14(3):164-172. Published online June 22, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0096
- 1,726 View
- 116 Download
- 1 Web of Science
- 1 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 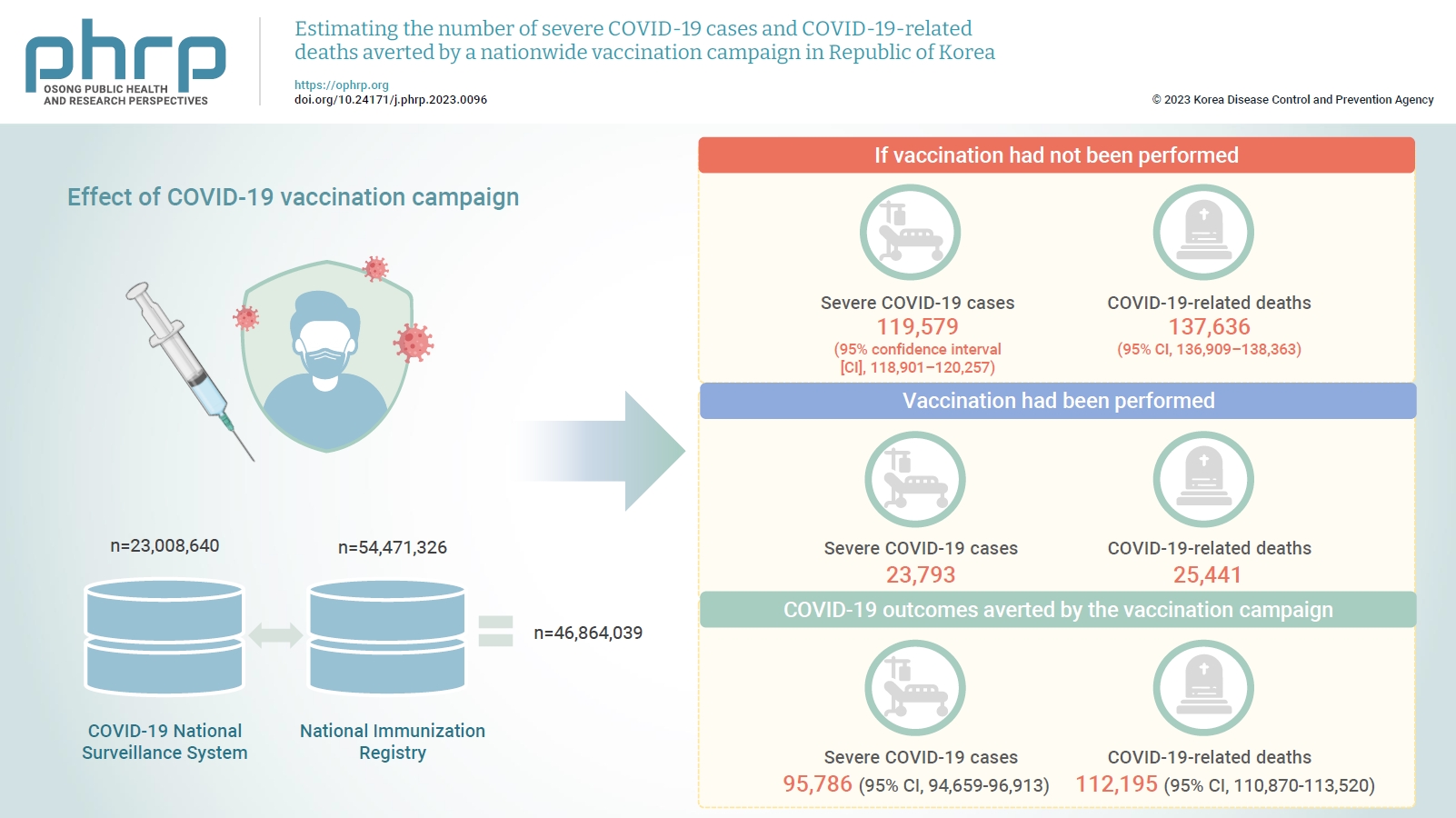
- Objectives
The Korea Disease Control and Prevention Agency promotes vaccination by regularly providing information on its benefits for reducing the severity of coronavirus disease 2019 (COVID-19). This study aimed to analyze the number of averted severe COVID-19 cases and COVID-19-related deaths by age group and quantify the impact of Republic of Korea’s nationwide vaccination campaign.
Methods
We analyzed an integrated database from the beginning of the vaccination campaign on February 26, 2021 to October 15, 2022. We estimated the cumulative number of severe cases and COVID-19-related deaths over time by comparing observed and estimated cases among unvaccinated and vaccinated groups using statistical modeling. We compared daily age-adjusted rates of severe cases and deaths in the unvaccinated group to those in the vaccinated group and calculated the susceptible population and proportion of vaccinated people by age.
Results
There were 23,793 severe cases and 25,441 deaths related to COVID-19. We estimated that 119,579 (95% confidence interval [CI], 118,901–120,257) severe COVID-19 cases and 137,636 (95% CI, 136,909–138,363) COVID-19-related deaths would have occurred if vaccination had not been performed. Therefore, 95,786 (95% CI, 94,659–96,913) severe cases and 112,195 (95% CI, 110,870–113,520) deaths were prevented as a result of the vaccination campaign.
Conclusion
We found that, if the nationwide COVID-19 vaccination campaign had not been implemented, the number of severe cases and deaths would have been at least 4 times higher. These findings suggest that Republic of Korea’s nationwide vaccination campaign reduced the number of severe cases and COVID-19 deaths. -
Citations
Citations to this article as recorded by- Comparative Effectiveness of COVID-19 Bivalent Versus Monovalent mRNA Vaccines in the Early Stage of Bivalent Vaccination in Korea: October 2022 to January 2023
Ryu Kyung Kim, Young June Choe, Eun Jung Jang, Chungman Chae, Ji Hae Hwang, Kil Hun Lee, Ji Ae Shim, Geun-Yong Kwon, Jae Young Lee, Young-Joon Park, Sang Won Lee, Donghyok Kwon
Journal of Korean Medical Science.2023;[Epub] CrossRef
- Comparative Effectiveness of COVID-19 Bivalent Versus Monovalent mRNA Vaccines in the Early Stage of Bivalent Vaccination in Korea: October 2022 to January 2023
Brief Report
- Temporal association between the age-specific incidence of Guillain-Barré syndrome and SARS-CoV-2 vaccination in Republic of Korea: a nationwide time-series correlation study
- Hyunju Lee, Donghyok Kwon, Seoncheol Park, Seung Ri Park, Darda Chung, Jongmok Ha
- Osong Public Health Res Perspect. 2023;14(3):224-231. Published online June 22, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0050
- 2,079 View
- 89 Download
- 1 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 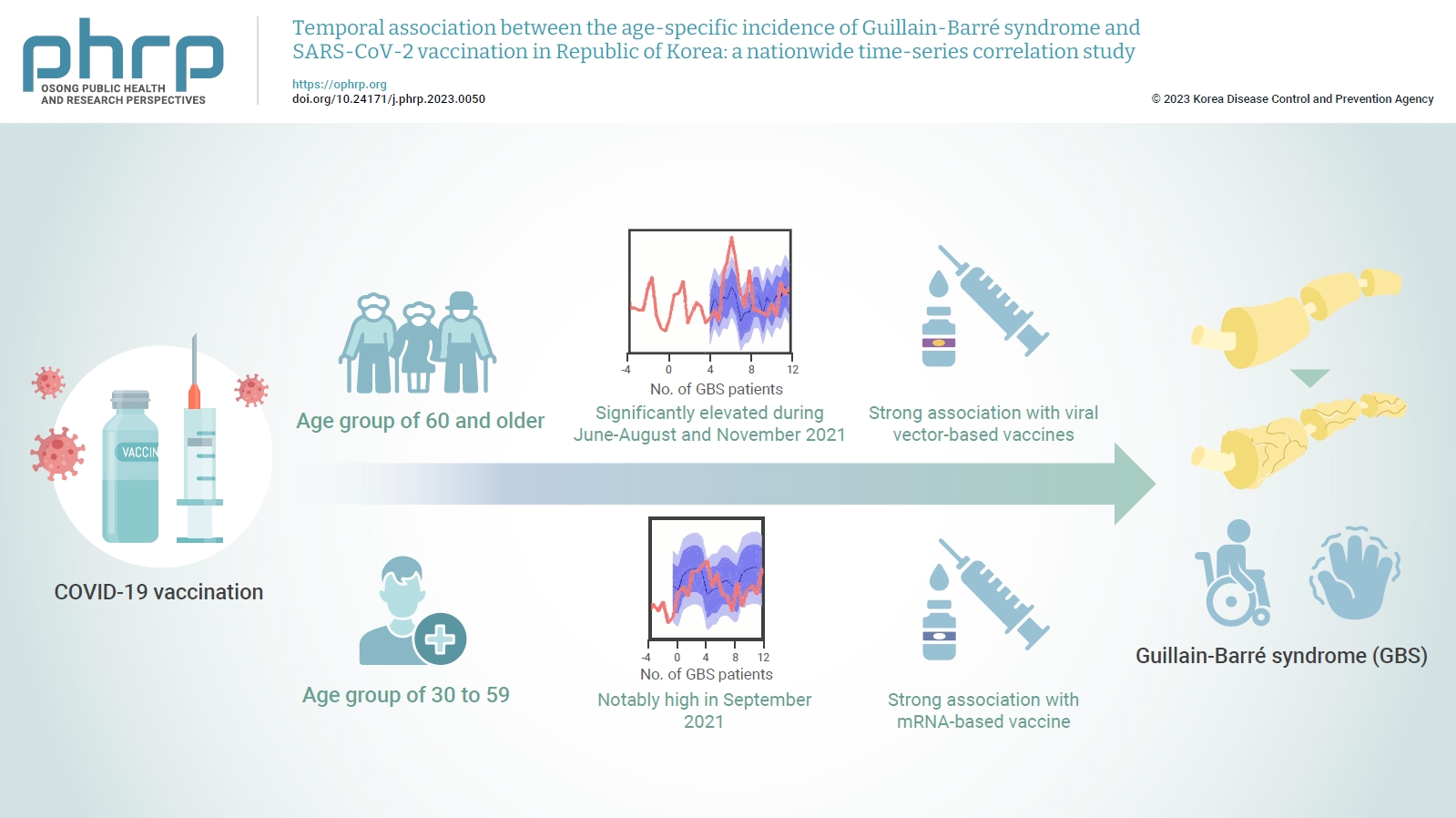
- Objectives
The incidence of Guillain-Barré syndrome (GBS) changed significantly during the coronavirus disease 2019 (COVID-19) pandemic. Emerging reports suggest that viral vector-based vaccines may be associated with an elevated risk of GBS.
Methods
In this nationwide time-series correlation study, we examined the age-specific incidence of GBS from January 2011 to August 2022, as well as data on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinations and infections from February 2021 to August 2022. We compared the forecasted estimates of age-specific GBS incidence, using the pre-SARS-CoV-2 period as a benchmark, with the actual incidence observed during the post-vaccination period of the pandemic. Furthermore, we assessed the temporal association between GBS, SARS-CoV-2 vaccinations, and COVID-19 for different age groups.
Results
In the age group of 60 and older, the rate ratio was significantly elevated during June-August and November 2021. A significant, strong positive association was observed between viral vector-based vaccines and GBS incidence trends in this age group (r=0.52, p=0.022). For the 30 to 59 years age group, the rate ratio was notably high in September 2021. A statistically significant, strong positive association was found between mRNA-based vaccines and GBS incidence in this age group (r=0.61, p=0.006).
Conclusion
Viral vector-based SARS-CoV-2 vaccines were found to be temporally associated with an increased risk of GBS, particularly in older adults. To minimize age-specific and biological mechanism-specific adverse events, future vaccination campaigns should adopt a more personalized approach, such as recommending homologous mRNA-based SARS-CoV-2 vaccines for older adults to reduce the heightened risk of GBS. -
Citations
Citations to this article as recorded by- mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions
Tamás Bakos, Tamás Mészáros, Gergely Tibor Kozma, Petra Berényi, Réka Facskó, Henriette Farkas, László Dézsi, Carlo Heirman, Stefaan de Koker, Raymond Schiffelers, Kathryn Anne Glatter, Tamás Radovits, Gábor Szénási, János Szebeni
International Journal of Molecular Sciences.2024; 25(7): 3595. CrossRef - Guillain–Barre syndrome following COVID-19 vaccination: a study of 70 case reports
Biki Kumar Sah, Zahra Fatima, Rajan Kumar Sah, Bushra Syed, Tulika Garg, Selia Chowdhury, Bikona Ghosh, Binita Kunwar, Anagha Shree, Vivek Kumar Sah, Anisha Raut
Annals of Medicine & Surgery.2024; 86(4): 2067. CrossRef
- mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions
Original Article
- Analysis of pregnant women with critically severe COVID-19 in Republic of Korea from February 2020 and December 2021
- Ji Joo Lee, Sang-Eun Lee, Yeonjung Kim, Young-Joon Park
- Osong Public Health Res Perspect. 2023;14(2):129-137. Published online April 5, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0025
- 1,295 View
- 58 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 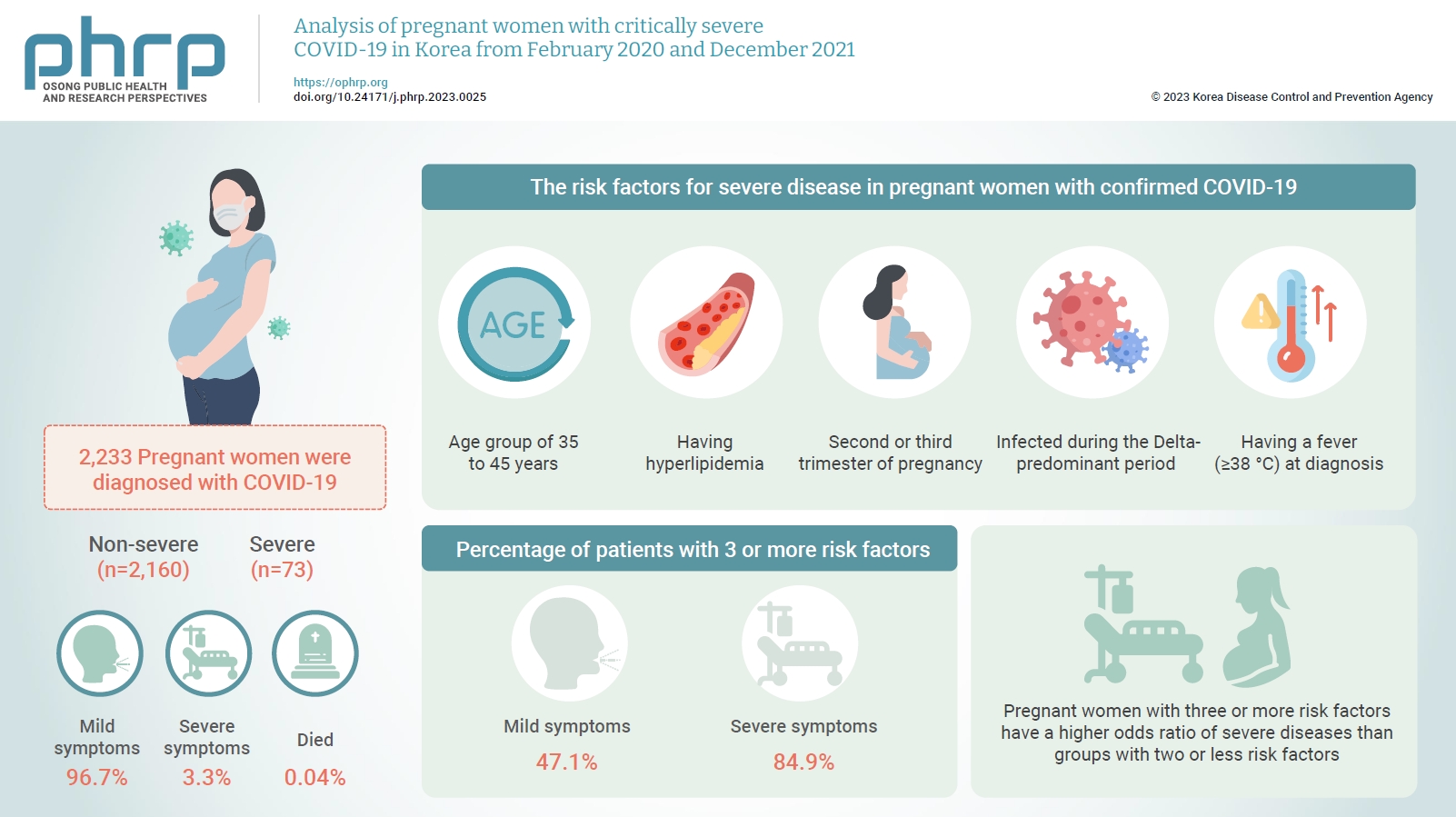
- Objectives
This study aimed to describe the characteristics and risk factors for severe disease in pregnant women infected with coronavirus disease 2019 (COVID-19) from the early days of the COVID-19 epidemic in Korea to the predominant period of the Delta variant.
Methods
A retrospective cohort study was conducted among pregnant women diagnosed with COVID-19 between February 2020 and December 2021. Logistic regression analysis was performed to compare severe and mild cases after adjusting for pregnant women’s age, nationality, infection route, outbreak area, infection period, symptoms, underlying disease, smoking status, trimester, and COVID-19 vaccination status.
Results
In total, 2,233 pregnant women were diagnosed with COVID-19 by December 2021. Among these, 96.7% had mild symptoms, 3.3% had severe symptoms, and 0.04% died. The risk factors for severe disease in pregnant women with confirmed COVID-19 were being in the age group of 35 to 45 years, having hyperlipidemia, being in the second or third trimester of pregnancy at the time of COVID-19 diagnosis, being infected during the Delta-predominant period, and having a fever (≥38 °C) at diagnosis. Furthermore, 47.1% of patients in the mild group and 84.9% of patients in the severe group had 3 or more risk factors.
Conclusion
Pregnant women with COVID-19 mainly experienced mild symptoms, but those with risk factors were at a higher risk of developing severe symptoms. Therefore, treatment and follow-up management should be thoroughly implemented.
Review Article
- SARS-CoV-2 in brief: from virus to prevention
- Hassan Karami, Zeinab Karimi, Negin Karami
- Osong Public Health Res Perspect. 2022;13(6):394-406. Published online November 28, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0155
- 2,829 View
- 96 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - The recent outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), ahighly transmissible virus with a likely animal origin, has posed major and unprecedentedchallenges to millions of lives across the affected nations of the world. This outbreak firstoccurred in China, and despite massive regional and global attempts shortly thereafter, itspread to other countries and caused millions of deaths worldwide. This review presents keyinformation about the characteristics of SARS-CoV-2 and its associated disease (namely,coronavirus disease 2019) and briefly discusses the origin of the virus. Herein, we also brieflysummarize the strategies used against viral spread and transmission.
-
Citations
Citations to this article as recorded by- Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic
Radu Lefter, Prairna Balyan, Ioana-Miruna Balmus, Abdellah Ech-Chahad, Ahmad Ali, Alin Ciobica, Antoneta Dacia Petroaie, Gabriela Halitchi, Bogdan Novac, Catalina Ionescu, Fatima Zahra Kamal
Microbiology Research.2024; 15(2): 525. CrossRef - Surveillance of endemic coronaviruses during the COVID‐19 pandemic in Iran, 2021–2022
Hassan Karami, Kaveh Sadeghi, Sevrin Zadheidar, Fatemeh Saadatmand, Negar Mirsalehi, Nima Hoveidi Ardestani, Shirin Kalantari, Mohammad Farahmand, Jila Yavarian, Talat Mokhtari‐Azad
Influenza and Other Respiratory Viruses.2023;[Epub] CrossRef
- Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic
Brief Report
- Presumed population immunity to SARS-CoV-2 in South Korea, April 2022
- Eun Jung Jang, Young June Choe, Seung Ah Choe, Yoo-Yeon Kim, Ryu Kyung Kim, Jia Kim, Do Sang Lim, Ju Hee Lee, Seonju Yi, Sangwon Lee, Young-Joon Park
- Osong Public Health Res Perspect. 2022;13(5):377-381. Published online October 14, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0209
- 2,354 View
- 71 Download
- 3 Web of Science
- 4 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 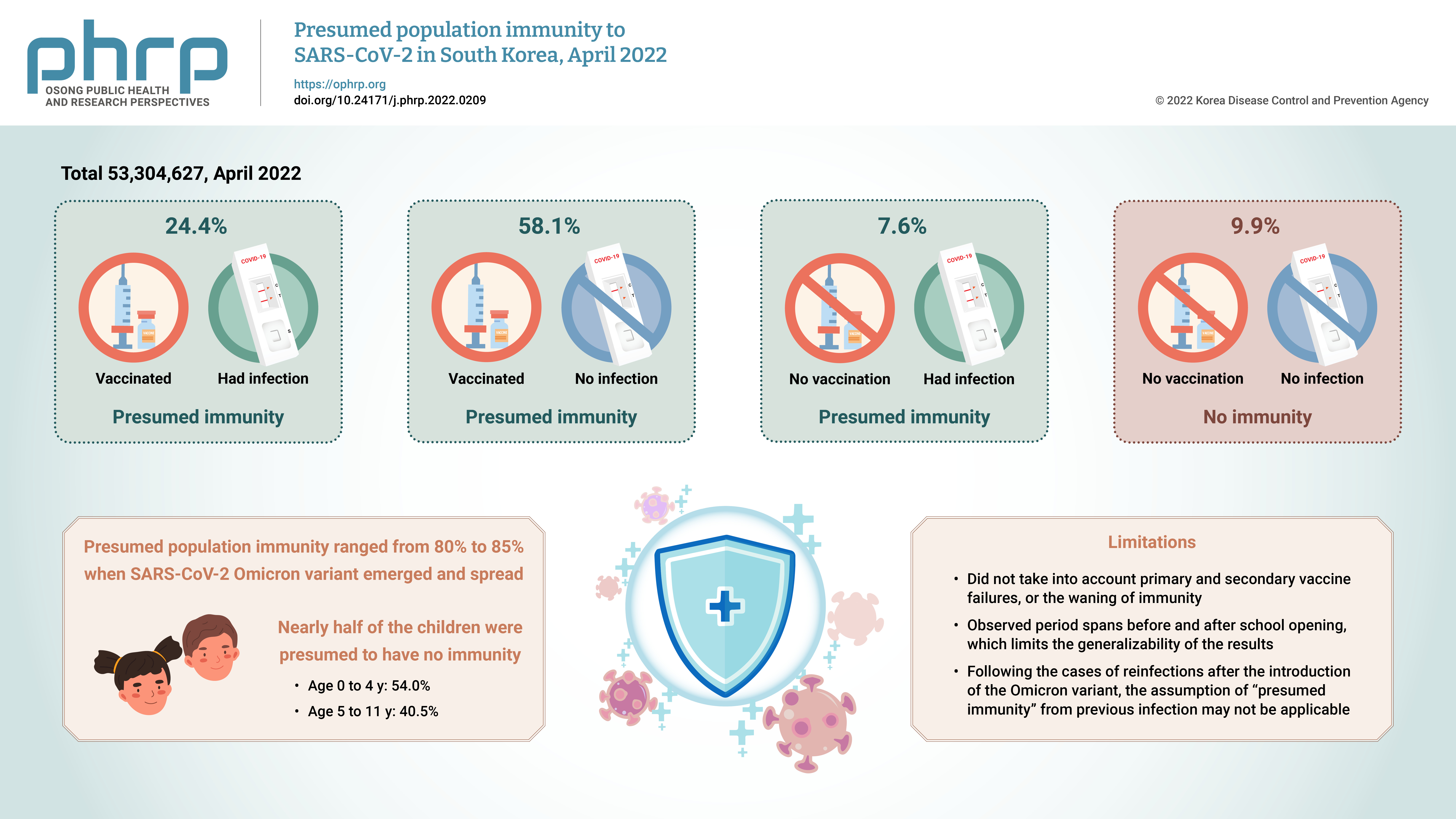
- Objectives
We estimated the overall and age-specific percentages of the Korean population with presumed immunity against severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) as of April 2022 using the national registry.
Methods
We used the national coronavirus disease 2019 (COVID-19) infection and vaccination registry from South Korea, as described to define individuals with a previous history of COVID-19 infection, vaccination, or both, as persons with presumed immunity.
Results
Of a total of 53,304,627 observed persons, 24.4% had vaccination and infection, 58.1% had vaccination and no infection, 7.6% had infection and no vaccination, and 9.9% had no immunity. The SARS-CoV-2 Omicron variant emerged at a time when the presumed population immunity ranged from 80% to 85%; however, nearly half of the children were presumed to have no immunity.
Conclusion
We report a gap in population immunity, with lower presumed protection in children than in adults. The approach presented in this work can provide valuable informed tools to assist vaccine policy-making at a national level. -
Citations
Citations to this article as recorded by- Realistic Estimation of COVID-19 Infection by Seroprevalence Surveillance of SARS-CoV-2 Antibodies: An Experience From Korea Metropolitan Area From January to May 2022
In Hwa Jeong, Jong-Hun Kim, Min-Jung Kwon, Jayoung Kim, Hee Jin Huh, Byoungguk Kim, Junewoo Lee, Jeong-hyun Nam, Eun-Suk Kang
Journal of Korean Medical Science.2024;[Epub] CrossRef - Epidemiology of Coronavirus Disease 2019 in Infants and Toddlers, Seoul, South Korea
JiWoo Sim, Euncheol Son, Young June Choe
Pediatric Infection & Vaccine.2024; 31(1): 94. CrossRef - Predicting adherence to COVID-19 preventive measures among South Korean adults aged 40 to 69 Years using the expanded health empowerment model
Su-Jung Nam, Tae-Young Pak
SSM - Population Health.2023; 22: 101411. CrossRef - Acute COVID-19 in unvaccinated children without a history of previous infection during the delta and omicron periods
Jee Min Kim, Ji Yoon Han, Seung Beom Han
Postgraduate Medicine.2023; 135(7): 727. CrossRef
- Realistic Estimation of COVID-19 Infection by Seroprevalence Surveillance of SARS-CoV-2 Antibodies: An Experience From Korea Metropolitan Area From January to May 2022
Original Articles
- Investigation of SARS-CoV-2 lineages and mutations circulating in a university-affiliated hospital in South Korea analyzed using Oxford Nanopore MinION sequencing
- Hyaekang Kim, Sung Hee Chung, Hyun Soo Kim, Han-Sung Kim, Wonkeun Song, Ki Ho Hong, Jae-Seok Kim
- Osong Public Health Res Perspect. 2022;13(5):360-369. Published online October 11, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0183
- 3,085 View
- 76 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Objectives
Despite the introduction of vaccines, treatments, and massive diagnostic testing, the evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has continued to overcome barriers that had slowed its previous spread. As the virus evolves towards increasing fitness, it is critical to continue monitoring the occurrence of new mutations that could evade human efforts to control them. Methods: We performed whole-genome sequencing using Oxford Nanopore MinION sequencing on 58 SARS-CoV-2 isolates collected during the ongoing coronavirus disease 2019 pandemic at a tertiary hospital in South Korea and tracked the emergence of mutations responsible for massive spikes in South Korea. Results: The differences among lineages were more pronounced in the spike gene, especially in the receptor-binding domain (RBD), than in other genes. Those RBD mutations could compromise neutralization by antibodies elicited by vaccination or previous infections. We also reported multiple incidences of Omicron variants carrying mutations that could impair the diagnostic sensitivity of reverse transcription-polymerase chain reaction-based testing. Conclusion: These results provide an understanding of the temporal changes of variants and mutations that have been circulating in South Korea and their potential impacts on antigenicity, therapeutics, and diagnostic escape of the virus. We also showed that the utilization of the nanopore sequencing platform and the ARTIC workf low can provide convenient and accurate SARS-CoV-2 genomic surveillance even at a single hospital. -
Citations
Citations to this article as recorded by- Understanding large scale sequencing datasets through changes to protein folding
David Shorthouse, Harris Lister, Gemma S Freeman, Benjamin A Hall
Briefings in Functional Genomics.2024;[Epub] CrossRef - Molecular epidemiology of SARS‐CoV‐2 in Mongolia, first experience with nanopore sequencing in lower‐ and middle‐income countries setting
Munkhtuya Erendereg, Suvd Tumurbaatar, Otgonjargal Byambaa, Gerelmaa Enebish, Natsagdorj Burged, Tungalag Khurelsukh, Nomin‐Erdene Baatar, Badmaarag Munkhjin, Jargaltulga Ulziijargal, Anuujin Gantumur, Oyunbaatar Altanbayar, Ochbadrakh Batjargal, Delgermu
Immunity, Inflammation and Disease.2023;[Epub] CrossRef
- Understanding large scale sequencing datasets through changes to protein folding
- Seroprevalence of immunoglobulin G antibodies against SARS-CoV-2 in children and adolescents in Delhi, India, from January to October 2021: a repeated cross-sectional analysis
- Pragya Sharma, Saurav Basu, Suruchi Mishra, Mongjam Meghachandra Singh
- Osong Public Health Res Perspect. 2022;13(3):184-190. Published online June 10, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0014
- 5,207 View
- 77 Download
- 3 Web of Science
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Objectives
The aim of this study was to assess changes in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) seroprevalence among children and adolescents in Delhi, India from January 2021 to October 2021. Methods: This was a repeated cross-sectional analysis of participants aged 5 to 17 years from 2 SARS-CoV-2 seroprevalence surveys conducted in Delhi, India during January 2021 and September to October 2021. Anti-SARS-CoV-2 IgG antibodies were detected by using the VITROS assay (90% sensitivity, 100% specificity). Results: The seroprevalence among 5- to 17-year-old school-age children and adolescents increased from 52.8% (95% confidence interval [CI], 51.3%−54.3%) in January 2021 to 81.8% (95% CI, 80.9%−82.6%) in September to October 2021. The assay-adjusted seroprevalence was 90.8% (95% CI, 89.8%−91.7%). Seropositivity positively correlated with participants’ age (p<0.001), but not sex (p=0.388). A signal to cut-off ratio ≥4.00, correlating with the presence of neutralization antibodies, was observed in 4,814 (57.9%) participants. Conclusion: The high percentage of seroconversion among children and adolescents indicates the presence of natural infection-induced immunity from past exposure to the SARS-CoV-2 virus. However, the lack of hybrid immunity and the concomitant likelihood of lower levels of neutralization antibodies than in adults due to the absence of vaccination warrants careful monitoring and surveillance of infection risk and disease severity from newer and emergent variants. -
Citations
Citations to this article as recorded by- Severe Acute Hepatitis of Unknown Etiology Presenting as Pediatric Acute Liver Failure: Analysis of Likely Etiology, Clinical Course and Outcome
Bikrant B. Lal, Vikrant Sood, Ekta Gupta, Reshu Agarwal, Rajeev Khanna, Seema Alam
Journal of Clinical and Experimental Hepatology.2023; 13(5): 912. CrossRef - Anti-SARS-CoV-2 antibody kinetics up to 6 months of follow-up: Result from a nation-wide population-based, age stratified sero-epidemiological prospective cohort study in India
Puneet Misra, Arvind Kumar Singh, Baijayantimala Mishra, Bijayini Behera, Binod Kumar Patro, Guruprasad R. Medigeshi, Hari Shanker Joshi, Mohammad Ahmad, Pradeep Kumar Chaturvedi, Palanivel Chinnakali, Partha Haldar, Mohan Bairwa, Pradeep Kharya, Rahul Dh
PLOS ONE.2023; 18(12): e0287807. CrossRef - Seroprevalence of SARS CoV-2 among children after the second surge (June 2021) in a rural district of South India: Findings and lessons from a population-based survey
Carolin Elizabeth George, Leeberk Raja Inbaraj, Shon Rajukutty, Roshni Florina Joan, Sangeetha Muthuraj, Sindhulina Chandrasingh
Frontiers in Pediatrics.2022;[Epub] CrossRef
- Severe Acute Hepatitis of Unknown Etiology Presenting as Pediatric Acute Liver Failure: Analysis of Likely Etiology, Clinical Course and Outcome
Review Article
- Immune-related therapeutics: an update on antiviral drugs and vaccines to tackle the COVID-19 pandemic
- Iqra Mir, Sania Aamir, Syed Rizwan Hussain Shah, Muhammad Shahid, Iram Amin, Samia Afzal, Amjad Nawaz, Muhammad Umer Khan, Muhammad Idrees
- Osong Public Health Res Perspect. 2022;13(2):84-100. Published online April 27, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0024
- 5,117 View
- 97 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 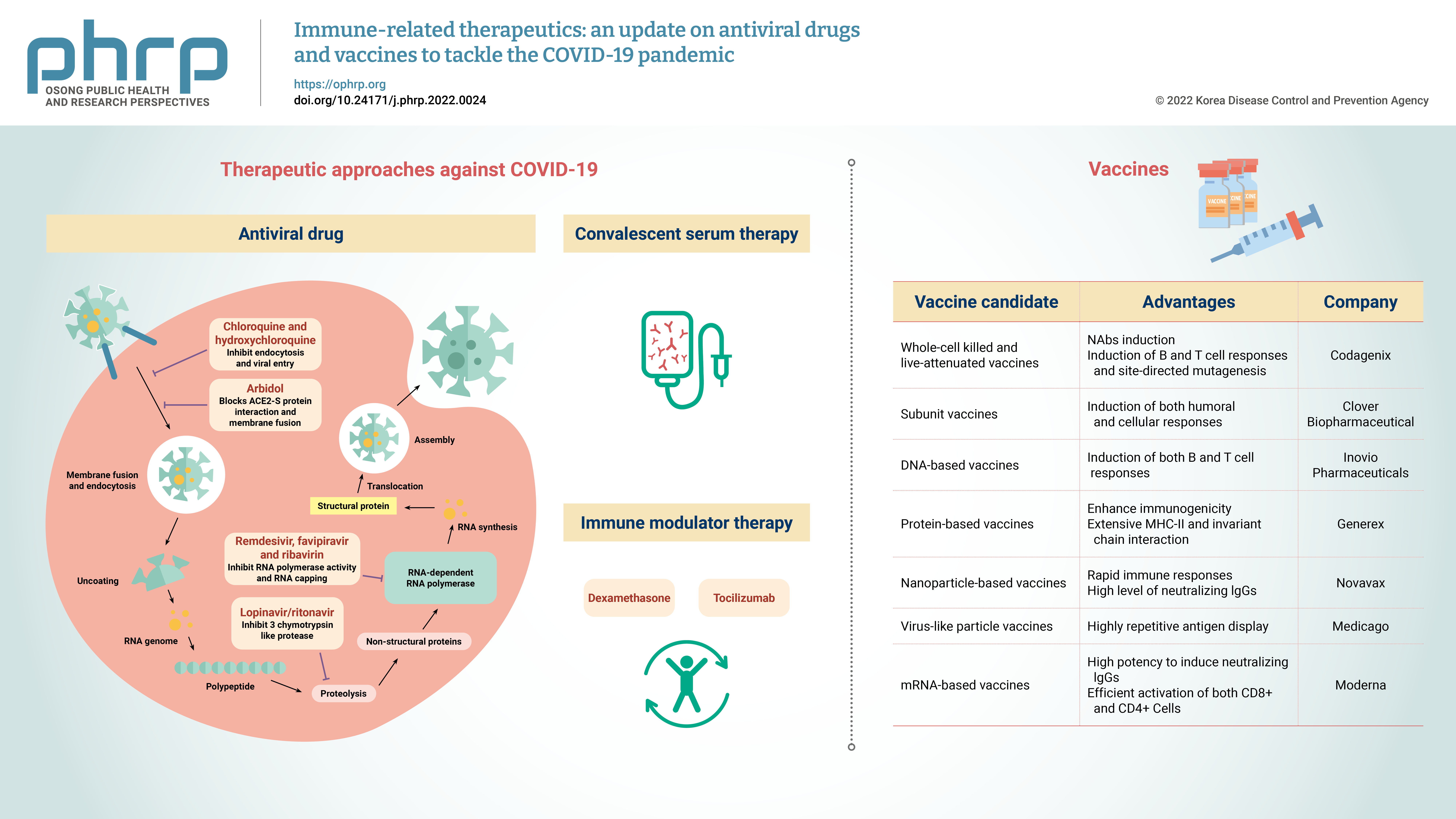
- The coronavirus disease 2019 (COVID-19) pandemic rapidly spread globally. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, is a positive-sense single-stranded RNA virus with a reported fatality rate ranging from 1% to 7%, and people with immune-compromised conditions, children, and older adults are particularly vulnerable. Respiratory failure and cytokine storm-induced multiple organ failure are the major causes of death. This article highlights the innate and adaptive immune mechanisms of host cells activated in response to SARS-CoV-2 infection and possible therapeutic approaches against COVID-19. Some potential drugs proven to be effective for other viral diseases are under clinical trials now for use against COVID-19. Examples include inhibitors of RNA-dependent RNA polymerase (remdesivir, favipiravir, ribavirin), viral protein synthesis (ivermectin, lopinavir/ ritonavir), and fusion of the viral membrane with host cells (chloroquine, hydroxychloroquine, nitazoxanide, and umifenovir). This article also presents the intellectual groundwork for the ongoing development of vaccines in preclinical and clinical trials, explaining potential candidates (live attenuated-whole virus vaccines, inactivated vaccines, subunit vaccines, DNAbased vaccines, protein-based vaccines, nanoparticle-based vaccines, virus-like particles and mRNA-based vaccines). Designing and developing an effective vaccine (both prophylactic and therapeutic) would be a long-term solution and the most effective way to eliminate the COVID-19 pandemic.



 First
First Prev
Prev


